Mio Jiang 1, Govind R. Rajan 2
Author affiliations:
Laparoscopic surgery has become a cornerstone of modern medicine, emerging as one of the most frequently performed procedures in the most of the world. It serves as a minimally invasive technique used for a range of diagnostic and therapeutic interventions, including cholecystectomy, hysterectomy, and tissue biopsies. It needs insufflation of the carbon dioxide (CO2) peritoneal cavity to offer space in the abdominal cavity. Veress needle technique being one of the most commonly used, which may injure abdominal contents and blood vessels. This case report is about the inadvertent intrahepatic entry of the Veress needle.
Keywords: Cholecystectomy; Laparoscopic surgery; Veress needle
Citation: Jiang M, Rajan GR. Managing carbon dioxide embolism in hepatobiliary laparoscopic surgery: a case report. Anaesth. pain intensive care 2025;29(2):345-349. DOI: 10.35975/apic.v29i2.2727
Laparoscopic surgery has become a cornerstone of modern medicine, emerging as one of the most frequently performed procedures in the United States. It serves as a minimally invasive technique used for a range of diagnostic and therapeutic interventions, including cholecystectomy, hysterectomy, and tissue biopsies. Compared to conventional open surgeries, laparoscopic procedures have shown benefits such as reduced hospital stays, fewer wound infections, less reported pain, and faster recovery times.1
Although relatively rare, a small percentage of patients experience severe complications during laparoscopic surgery, often related to the initial entry into the abdomen.2 A pneumoperitoneum is created by insufflating the peritoneal cavity with carbon dioxide (CO2) and is essential for providing the necessary visualization and working space for the procedure. Several methods exist for gaining initial access to the peritoneal cavity, with the Veress needle technique being one of the most commonly used. Among the potential complications, injury to intra-abdominal vasculature during Veress needle insertion is one of the most serious ones. Such injuries can lead to a range of adverse outcomes, including significant hemorrhage, organ injury, and gas embolism.
We present a case of CO2 embolism following Veress needle insufflation during a hepatobiliary laparoscopic procedure due to inadvertent intrahepatic placement of Veress Needle. Informed consent was obtained from the patient, and all patient information was handled per institutional Health Insurance Portability and Accountability Act (HIPAA) requirements.
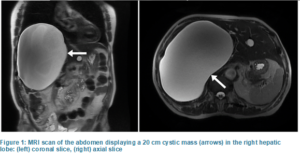
A 79-year-old male with a weight of 63 kg and body mass index of 21 kg/m2 with a large hepatic lesion presented for a laparoscopic hepatic cystectomy and cholecystectomy. His liver mass was found incidentally with no associated symptoms. A magnetic resonance imaging (MRI) scan of the abdomen revealed a 20 cm cystic mass occupying the entire right hepatic lobe, as displayed in Figure 1. His past medical history included hypertension, a thoracic aortic aneurysm without rupture, and benign prostatic hyperplasia, for which his outpatient medications included losartan, finasteride, and tamsulosin. Cardiology consultation pre-operatively reported a negative exercise stress test and an electrocardiogram (ECG) showing sinus rhythm with an average heart rate of 50, prolonged PR interval, and an old inferior infarct.
Preoperatively, the patient received midazolam 2 mg intravenously (IV) and was positioned supine. General anesthesia was induced with IV propofol 100 mg, fentanyl 50 µg, rocuronium 70 mg, and lidocaine 70 mg. Intubation was achieved without difficulty. Volatile sevoflurane was administered to maintain adequate anesthetic depth. After surgical site preparation and time-out, empiric antibiotics ceftriaxone 2 g IV and metronidazole 500 mg IVPB were administered.
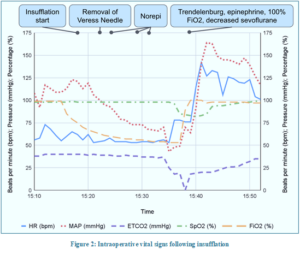
Before Veress needle insertion, adequate neuromuscular blockade was confirmed, and mechanical ventilation was temporarily held to minimize diaphragmatic movement. The Veress needle was inserted at Palmer's point in the left upper quadrant, and the saline drop test confirmed the correct placement. Intraoperative parameters are displayed in Table 1 below. CO2 insufflation commenced with an opening pressure of 7 mmHg, increasing to 14 mmHg. Ventilation settings included a set tidal volume of 475 mL, a set respiration rate of 10 breaths per minute, and 5 cmH2O positive end-expiratory pressure (PEEP). Shortly after the Veress needle was removed, the patient developed precipitous hypotension. Norepinephrine 4 µg IV was administered. Over the next few minutes, end-tidal carbon dioxide (EtCO2) decreased to 0 mmHg, and simultaneously, oxygen saturation (SpO2) started falling to 83%. Mean arterial blood pressure (MAP) declined despite two additional 4 µg norepinephrine boluses. The patient was placed in the steep Trendelenburg position to improve venous return, epinephrine 100 µg IV was administered, ventilated with a 100% oxygen; sevoflurane administration was temporarily turned down.
These interventions stabilized the patient's hemodynamic status within the next few minutes. One hour after the patient's vitals returned to baseline, arterial blood gas (ABG) analysis revealed a pH 7.296, partial pressure of CO2 (PaCO2) 45.4 mmHg, and partial pressure of oxygen (PaO2) 190 mmHg. Subsequent ABG analysis one hour later revealed a pH 7.332, PaCO2 35.3 mmHg, and PaO2 199 mmHg.
We believe the Veress needle inadvertently entered the left lobe of the hepatic parenchyma; subsequently, with the initial CO2 pressure, the needle entered the hepatic sinusoids and eventually made its way into the right heart, leading to CO2 embolism (Figure 2).
Realizing the difficulty encountered with laparoscopic visualization caused by the massive hepatic cyst, a decision was made to convert to an open procedure. A midline laparotomy was performed, and the point of left hepatic lobe injury caused by the Veress needle was identified. The hepatic cyst was drained, and the capsule was excised. The cholecystectomy was completed without complications. The patient was extubated and transferred to the post-anesthesia care unit in a stable condition for continuous monitoring for delayed complications. He was discharged on postoperative day five without any further incident.
The most plausible cause of this patient's perioperative episode of severe hypotension, hypoxia, and abrupt reduction in EtCO2 was a gas embolism. This complication likely arose from the initial attempt to create pneumoperitoneum using a Veress needle at the Palmer point. Although the proximity of the displaced left lobe of the liver to the Palmer point was evident on the CT of the abdomen, we failed to recognize its clinical significance due to the presence of the large hepatic cyst, leading to the inadvertent puncture of the liver. The liver's extensive vascular supply makes it particularly susceptible to CO2 entrainment during insufflation if a vessel is disrupted. Given that the hepatic vasculature drains directly into the inferior vena cava, the likelihood of gas embolism may be inherently higher during procedures involving the liver. This increased risk is suggested by the higher incidence of gas embolisms during laparoscopic hepatectomy, with a rate of 0.2 to 1.5% compared to 0.15% in general laparoscopic surgeries.3 Additionally, a small serosal tear on the stomach indicates the possibility of intra-abdominal damage from the placement of the Veress needle. Following a return of vitals to baseline, ABG analysis showed mild respiratory acidosis, consistent with a ventilation-perfusion mismatch, which improved subsequently.
Gas emboli during insufflation primarily occur through two mechanisms: 1) direct injection of gas into the intra-abdominal vasculature due to improper placement of the Veress needle, or more commonly, 2) gas entering damaged vascular structures, leading to a delayed onset embolism.4 In this patient, there was no overt evidence of vascular damage indicative of Veress needle misplacement, and the hemodynamic instability developed gradually over ten minutes. The positive saline drop test further supported the Veress needle's correct placement within the abdominal cavity, pointing to the second mechanism of embolism formation. One possible explanation is that diaphragmatic movement during inspiration may have caused the liver to shift downwards, allowing gas to gradually enter the bloodstream, resulting in a delayed onset of embolism symptoms.
Although a clinically significant CO2 embolism is rare, it is a life-threatening complication of laparoscopic surgery with a mortality rate of 28%.4 Early detection is critical for patient survival. Several papers have documented success while using ultrasound guidance while placing the Veress needle in particularly high-risk patients, such as morbidly obese patients. Due to its high sensitivity, a transesophageal echocardiogram (TEE) is considered the gold standard for detecting gas emboli. However, its routine use is limited by high cost and the need for constant operator attention.5 Transesophageal Doppler better balances high sensitivity with lower operation costs, making it a feasible alternative to TEE.5 EtCO2 monitoring, though less sensitive than TTE and Doppler, is non-invasive, widely available, and provides real-time feedback that can prompt immediate clinical response.6 Pulmonary artery pressure monitoring is another method used to identify gas emboli; however, pulmonary artery catheters are associated with increased complications, such as arrhythmias, catheter misplacement, and rarely pulmonary artery rupture. To mitigate these risks, less invasive methods are often preferred. A 'mill-wheel' murmur may be identified on esophageal or precordial auscultation, but this sign has yielded inconsistent results.5 Closely monitoring hemodynamic variables and ECG changes is essential for identifying a possible CO2 embolism.3 In this patient, a sudden decrease in MAP, EtCO2, and SpO2 raised concern for a gas embolism. While these signs were sufficient for initiating treatment in this patient, utilizing additional detection techniques such as TEE could have facilitated a quicker diagnosis and intervention. Several studies have documented the successful use of ultrasound guidance when placing the Veress needle in high-risk patients, such as those with morbid obesity, which may also be beneficial in similar scenarios to improve placement accuracy and reduce complications.7
Timely treatment is crucial to avoiding lethal complications from suspected CO2 emboli. Without associated symptoms, a small amount of venous insufflation may be managed with observation. Studies have demonstrated that the lethal dose of CO2 for a 70 kg human ranges from 600 mL to 1750 mL; thus, clinical symptoms should guide the management.8 For patients with hemodynamic instability, essential steps include immediate cessation of CO2 insufflation and deflation of the pneumoperitoneum. Placing the patient in Trendelenburg (supine with head down) or Durant (left lateral decubitus with head down) position prevents further gas entry into the pulmonary artery by allowing the gas to rise to the apex of the right ventricle.4,5 Ventilation with 100% oxygen is vital to wash out CO2 and improve hypoxia and ventilation-perfusion mismatch.5,6 In severe cases, a central venous line or pulmonary artery catheter may be necessary for invasive monitoring and aspiration of the obstructing gas.4,6 Inotropes, vasopressors, and aggressive fluid resuscitation may be required to support cardiac output during resuscitation.2 Cardiopulmonary resuscitation should be initiated when necessary. The patient's vital signs returned to baseline quickly with Trendelenburg positioning, ventilation with 100% oxygen, and epinephrine administration. The rapid treatment potentially prevented fatal outcomes associated with prolonged hypoxia and hypotension, including arrhythmias and cardiovascular collapse.
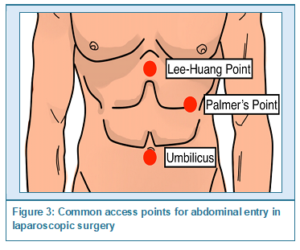
Preventive measures are paramount in minimizing the risk of CO2 embolism. The most common sites of initial entry for abdominal laparoscopic surgeries include the umbilicus and Palmer's point, located three cm below the left costal margin in the midclavicular line, illustrated in Figure 3. The umbilicus is often utilized due to its central location and thin subcutaneous fat layer, facilitating easier access to the peritoneal cavity.9 Palmer's point is recommended for patients with previous abdominal surgeries or known adhesions, umbilical pathologies such as hernias, or three failed trans-umbilical attempts. Relative contraindications include a large abdominal mass, hepatomegaly, or splenomegaly due to an increased risk of visceral injury. Given the location and size of the hepatic mass in this patient, both the umbilicus and Palmer's point posed increased risks of intraoperative complications. The patient's large hepatic mass displaced other organs, necessitating a careful review of existing patient imaging to determine the best entry site. Some studies recommend reducing insufflation pressure to 12 mmHg from 15 mmHg to reduce the incidence of CO2 emboli; however, this technique has not been shown to be effective for laparoscopic cholecystectomies.10 Alternative entry techniques, such as the open Hasson technique, may provide safer outcomes and should be considered to minimize risks.4 Patient positioning also plays a role in reducing the risk of gas embolism. Implementing the reverse Trendelenburg position (supine with head elevated) in certain pelvic and gynecologic procedures has been shown to decrease the incidence of gas embolism.5 The Trendelenburg position may help reduce the risk during specific procedures by increasing central venous pressure, thereby limiting gas entrainment and slowing the migration of existing CO2 emboli within the venous circulation. Additionally, applying PEEP during mechanical ventilation may reduce the pressure gradient between open vessels and the heart, decreasing the likelihood of gas entering the circulatory system. Further research is needed to determine optimal procedures for avoiding gas emboli and vascular damage.
In summary, we described a patient with a large hepatic lesion who developed a CO2 embolism after insufflation for laparoscopic surgery. In this case, the prompt clinical diagnosis based on the rapid hemodynamic instability characterized by a decrease in MAP and a subsequent decline in EtCO2 and SpO2 facilitated timely intervention. This case highlights the importance of vigilance, early detection, and immediate management in preventing a potentially fatal surgical complication.
Although comparatively rare, CO2 embolism is a significant complication of laparoscopic surgery, among more commonly encountered complications such as hypotension and bradycardia. Increased caution is imperative during hepatobiliary surgeries due to the liver’s extensive vascular supply. Considering the sparsity of information currently available, further research is warranted.
5. Conflict of interest
The authors declare no conflicts of interest.
6. Ethical considerations
Written consent was obtained from the patient to publish this case report in the academic interest.
7. Authors contribution
Both authors took part in the conduct of this case, literature search and preparation of this manuscript.
Author affiliations:
- Mio Jiang, Department of Anesthesiology & Perioperative Care, University of California, Orange, CA 92868, USA; Email: mcjiang@hs.uci.edu, {ORCID:0000-0001-8838-9468}
- Govind R. Rajan, Department of Anesthesiology & Perioperative Care, University of California, Orange, CA 92868, USA; Email: grajan@hs.uci.edu
ABSTRACT
Laparoscopic surgery has become a cornerstone of modern medicine, emerging as one of the most frequently performed procedures in the most of the world. It serves as a minimally invasive technique used for a range of diagnostic and therapeutic interventions, including cholecystectomy, hysterectomy, and tissue biopsies. It needs insufflation of the carbon dioxide (CO2) peritoneal cavity to offer space in the abdominal cavity. Veress needle technique being one of the most commonly used, which may injure abdominal contents and blood vessels. This case report is about the inadvertent intrahepatic entry of the Veress needle.
Keywords: Cholecystectomy; Laparoscopic surgery; Veress needle
Citation: Jiang M, Rajan GR. Managing carbon dioxide embolism in hepatobiliary laparoscopic surgery: a case report. Anaesth. pain intensive care 2025;29(2):345-349. DOI: 10.35975/apic.v29i2.2727
1. INTRODUCTION
Laparoscopic surgery has become a cornerstone of modern medicine, emerging as one of the most frequently performed procedures in the United States. It serves as a minimally invasive technique used for a range of diagnostic and therapeutic interventions, including cholecystectomy, hysterectomy, and tissue biopsies. Compared to conventional open surgeries, laparoscopic procedures have shown benefits such as reduced hospital stays, fewer wound infections, less reported pain, and faster recovery times.1
Although relatively rare, a small percentage of patients experience severe complications during laparoscopic surgery, often related to the initial entry into the abdomen.2 A pneumoperitoneum is created by insufflating the peritoneal cavity with carbon dioxide (CO2) and is essential for providing the necessary visualization and working space for the procedure. Several methods exist for gaining initial access to the peritoneal cavity, with the Veress needle technique being one of the most commonly used. Among the potential complications, injury to intra-abdominal vasculature during Veress needle insertion is one of the most serious ones. Such injuries can lead to a range of adverse outcomes, including significant hemorrhage, organ injury, and gas embolism.
We present a case of CO2 embolism following Veress needle insufflation during a hepatobiliary laparoscopic procedure due to inadvertent intrahepatic placement of Veress Needle. Informed consent was obtained from the patient, and all patient information was handled per institutional Health Insurance Portability and Accountability Act (HIPAA) requirements.
2. CASE REPORT
A 79-year-old male with a weight of 63 kg and body mass index of 21 kg/m2 with a large hepatic lesion presented for a laparoscopic hepatic cystectomy and cholecystectomy. His liver mass was found incidentally with no associated symptoms. A magnetic resonance imaging (MRI) scan of the abdomen revealed a 20 cm cystic mass occupying the entire right hepatic lobe, as displayed in Figure 1. His past medical history included hypertension, a thoracic aortic aneurysm without rupture, and benign prostatic hyperplasia, for which his outpatient medications included losartan, finasteride, and tamsulosin. Cardiology consultation pre-operatively reported a negative exercise stress test and an electrocardiogram (ECG) showing sinus rhythm with an average heart rate of 50, prolonged PR interval, and an old inferior infarct.

Preoperatively, the patient received midazolam 2 mg intravenously (IV) and was positioned supine. General anesthesia was induced with IV propofol 100 mg, fentanyl 50 µg, rocuronium 70 mg, and lidocaine 70 mg. Intubation was achieved without difficulty. Volatile sevoflurane was administered to maintain adequate anesthetic depth. After surgical site preparation and time-out, empiric antibiotics ceftriaxone 2 g IV and metronidazole 500 mg IVPB were administered.
Before Veress needle insertion, adequate neuromuscular blockade was confirmed, and mechanical ventilation was temporarily held to minimize diaphragmatic movement. The Veress needle was inserted at Palmer's point in the left upper quadrant, and the saline drop test confirmed the correct placement. Intraoperative parameters are displayed in Table 1 below. CO2 insufflation commenced with an opening pressure of 7 mmHg, increasing to 14 mmHg. Ventilation settings included a set tidal volume of 475 mL, a set respiration rate of 10 breaths per minute, and 5 cmH2O positive end-expiratory pressure (PEEP). Shortly after the Veress needle was removed, the patient developed precipitous hypotension. Norepinephrine 4 µg IV was administered. Over the next few minutes, end-tidal carbon dioxide (EtCO2) decreased to 0 mmHg, and simultaneously, oxygen saturation (SpO2) started falling to 83%. Mean arterial blood pressure (MAP) declined despite two additional 4 µg norepinephrine boluses. The patient was placed in the steep Trendelenburg position to improve venous return, epinephrine 100 µg IV was administered, ventilated with a 100% oxygen; sevoflurane administration was temporarily turned down.
These interventions stabilized the patient's hemodynamic status within the next few minutes. One hour after the patient's vitals returned to baseline, arterial blood gas (ABG) analysis revealed a pH 7.296, partial pressure of CO2 (PaCO2) 45.4 mmHg, and partial pressure of oxygen (PaO2) 190 mmHg. Subsequent ABG analysis one hour later revealed a pH 7.332, PaCO2 35.3 mmHg, and PaO2 199 mmHg.
We believe the Veress needle inadvertently entered the left lobe of the hepatic parenchyma; subsequently, with the initial CO2 pressure, the needle entered the hepatic sinusoids and eventually made its way into the right heart, leading to CO2 embolism (Figure 2).
Realizing the difficulty encountered with laparoscopic visualization caused by the massive hepatic cyst, a decision was made to convert to an open procedure. A midline laparotomy was performed, and the point of left hepatic lobe injury caused by the Veress needle was identified. The hepatic cyst was drained, and the capsule was excised. The cholecystectomy was completed without complications. The patient was extubated and transferred to the post-anesthesia care unit in a stable condition for continuous monitoring for delayed complications. He was discharged on postoperative day five without any further incident.
3. DISCUSSION
The most plausible cause of this patient's perioperative episode of severe hypotension, hypoxia, and abrupt reduction in EtCO2 was a gas embolism. This complication likely arose from the initial attempt to create pneumoperitoneum using a Veress needle at the Palmer point. Although the proximity of the displaced left lobe of the liver to the Palmer point was evident on the CT of the abdomen, we failed to recognize its clinical significance due to the presence of the large hepatic cyst, leading to the inadvertent puncture of the liver. The liver's extensive vascular supply makes it particularly susceptible to CO2 entrainment during insufflation if a vessel is disrupted. Given that the hepatic vasculature drains directly into the inferior vena cava, the likelihood of gas embolism may be inherently higher during procedures involving the liver. This increased risk is suggested by the higher incidence of gas embolisms during laparoscopic hepatectomy, with a rate of 0.2 to 1.5% compared to 0.15% in general laparoscopic surgeries.3 Additionally, a small serosal tear on the stomach indicates the possibility of intra-abdominal damage from the placement of the Veress needle. Following a return of vitals to baseline, ABG analysis showed mild respiratory acidosis, consistent with a ventilation-perfusion mismatch, which improved subsequently.

Gas emboli during insufflation primarily occur through two mechanisms: 1) direct injection of gas into the intra-abdominal vasculature due to improper placement of the Veress needle, or more commonly, 2) gas entering damaged vascular structures, leading to a delayed onset embolism.4 In this patient, there was no overt evidence of vascular damage indicative of Veress needle misplacement, and the hemodynamic instability developed gradually over ten minutes. The positive saline drop test further supported the Veress needle's correct placement within the abdominal cavity, pointing to the second mechanism of embolism formation. One possible explanation is that diaphragmatic movement during inspiration may have caused the liver to shift downwards, allowing gas to gradually enter the bloodstream, resulting in a delayed onset of embolism symptoms.
Although a clinically significant CO2 embolism is rare, it is a life-threatening complication of laparoscopic surgery with a mortality rate of 28%.4 Early detection is critical for patient survival. Several papers have documented success while using ultrasound guidance while placing the Veress needle in particularly high-risk patients, such as morbidly obese patients. Due to its high sensitivity, a transesophageal echocardiogram (TEE) is considered the gold standard for detecting gas emboli. However, its routine use is limited by high cost and the need for constant operator attention.5 Transesophageal Doppler better balances high sensitivity with lower operation costs, making it a feasible alternative to TEE.5 EtCO2 monitoring, though less sensitive than TTE and Doppler, is non-invasive, widely available, and provides real-time feedback that can prompt immediate clinical response.6 Pulmonary artery pressure monitoring is another method used to identify gas emboli; however, pulmonary artery catheters are associated with increased complications, such as arrhythmias, catheter misplacement, and rarely pulmonary artery rupture. To mitigate these risks, less invasive methods are often preferred. A 'mill-wheel' murmur may be identified on esophageal or precordial auscultation, but this sign has yielded inconsistent results.5 Closely monitoring hemodynamic variables and ECG changes is essential for identifying a possible CO2 embolism.3 In this patient, a sudden decrease in MAP, EtCO2, and SpO2 raised concern for a gas embolism. While these signs were sufficient for initiating treatment in this patient, utilizing additional detection techniques such as TEE could have facilitated a quicker diagnosis and intervention. Several studies have documented the successful use of ultrasound guidance when placing the Veress needle in high-risk patients, such as those with morbid obesity, which may also be beneficial in similar scenarios to improve placement accuracy and reduce complications.7
Timely treatment is crucial to avoiding lethal complications from suspected CO2 emboli. Without associated symptoms, a small amount of venous insufflation may be managed with observation. Studies have demonstrated that the lethal dose of CO2 for a 70 kg human ranges from 600 mL to 1750 mL; thus, clinical symptoms should guide the management.8 For patients with hemodynamic instability, essential steps include immediate cessation of CO2 insufflation and deflation of the pneumoperitoneum. Placing the patient in Trendelenburg (supine with head down) or Durant (left lateral decubitus with head down) position prevents further gas entry into the pulmonary artery by allowing the gas to rise to the apex of the right ventricle.4,5 Ventilation with 100% oxygen is vital to wash out CO2 and improve hypoxia and ventilation-perfusion mismatch.5,6 In severe cases, a central venous line or pulmonary artery catheter may be necessary for invasive monitoring and aspiration of the obstructing gas.4,6 Inotropes, vasopressors, and aggressive fluid resuscitation may be required to support cardiac output during resuscitation.2 Cardiopulmonary resuscitation should be initiated when necessary. The patient's vital signs returned to baseline quickly with Trendelenburg positioning, ventilation with 100% oxygen, and epinephrine administration. The rapid treatment potentially prevented fatal outcomes associated with prolonged hypoxia and hypotension, including arrhythmias and cardiovascular collapse.
Preventive measures are paramount in minimizing the risk of CO2 embolism. The most common sites of initial entry for abdominal laparoscopic surgeries include the umbilicus and Palmer's point, located three cm below the left costal margin in the midclavicular line, illustrated in Figure 3. The umbilicus is often utilized due to its central location and thin subcutaneous fat layer, facilitating easier access to the peritoneal cavity.9 Palmer's point is recommended for patients with previous abdominal surgeries or known adhesions, umbilical pathologies such as hernias, or three failed trans-umbilical attempts. Relative contraindications include a large abdominal mass, hepatomegaly, or splenomegaly due to an increased risk of visceral injury. Given the location and size of the hepatic mass in this patient, both the umbilicus and Palmer's point posed increased risks of intraoperative complications. The patient's large hepatic mass displaced other organs, necessitating a careful review of existing patient imaging to determine the best entry site. Some studies recommend reducing insufflation pressure to 12 mmHg from 15 mmHg to reduce the incidence of CO2 emboli; however, this technique has not been shown to be effective for laparoscopic cholecystectomies.10 Alternative entry techniques, such as the open Hasson technique, may provide safer outcomes and should be considered to minimize risks.4 Patient positioning also plays a role in reducing the risk of gas embolism. Implementing the reverse Trendelenburg position (supine with head elevated) in certain pelvic and gynecologic procedures has been shown to decrease the incidence of gas embolism.5 The Trendelenburg position may help reduce the risk during specific procedures by increasing central venous pressure, thereby limiting gas entrainment and slowing the migration of existing CO2 emboli within the venous circulation. Additionally, applying PEEP during mechanical ventilation may reduce the pressure gradient between open vessels and the heart, decreasing the likelihood of gas entering the circulatory system. Further research is needed to determine optimal procedures for avoiding gas emboli and vascular damage.

4. CONCLUSION
In summary, we described a patient with a large hepatic lesion who developed a CO2 embolism after insufflation for laparoscopic surgery. In this case, the prompt clinical diagnosis based on the rapid hemodynamic instability characterized by a decrease in MAP and a subsequent decline in EtCO2 and SpO2 facilitated timely intervention. This case highlights the importance of vigilance, early detection, and immediate management in preventing a potentially fatal surgical complication.
Although comparatively rare, CO2 embolism is a significant complication of laparoscopic surgery, among more commonly encountered complications such as hypotension and bradycardia. Increased caution is imperative during hepatobiliary surgeries due to the liver’s extensive vascular supply. Considering the sparsity of information currently available, further research is warranted.
5. Conflict of interest
The authors declare no conflicts of interest.
6. Ethical considerations
Written consent was obtained from the patient to publish this case report in the academic interest.
7. Authors contribution
Both authors took part in the conduct of this case, literature search and preparation of this manuscript.
8. REFERENCES
- Buia A, Stockhausen F, Hanisch E. Laparoscopic surgery: a qualified systematic review. World J Methodol. 2015;5(4):238-54. [PubMed] DOI: 5662/wjm.v5.i4.238
- Kindel T, Latchana N, Swaroop M, Chaudhry UI, Noria SF, Choron RL, et al. Laparoscopy in trauma: an overview of complications and related topics. Int J Crit Illn Inj Sci. 2015;5(3):196-205. [PubMed] DOI: 4103/2229-5151.165004
- Shimizu K, Usuda M, Kakizaki Y, Narita T, Suzuki O, Fukuoka K. Cerebral infarction by paradoxical gas embolism detected after laparoscopic partial hepatectomy with an insufflation management system: a case report. Surg Case Rep. 2023;9(1):34. [PubMed] DOI: 1186/s40792-023-01611-0
- Gutt CN, Oniu T, Mehrabi A, Schemmer P, Kashfi A, Kraus T, et al. Circulatory and respiratory complications of carbon dioxide insufflation. Dig Surg. 2004;21(2):95-105. [PubMed] DOI: 1159/000077038
- Park EY, Kwon JY, Kim KJ. Carbon dioxide embolism during laparoscopic surgery. Yonsei Med J. 2012;53(3):459-66. [PubMed] DOI: 3349/ymj.2012.53.3.459
- Upadhyay PS. Near fatal carbon dioxide embolism during laparoscopy and its successful aspiration using ultrasound guided catheter. J Anesth Intensive Care Med. 2016;1. DOI: 19080/JAICM.2016.01.555564
- Cakir T, Tuney D, Esmaeilzadem S, Aktan AO. Safe Veress needle insertion. J Hepatobiliary Pancreat Surg. 2006;13(3):225-7. [PubMed] DOI: 1007/s00534-005-1024-x
- Davolio MC, Pizzirani M, Vecchio S, Tore E, Strocchi M, Melotti G, et al. Medico-legal implications for carbon dioxide embolism during laparoscopic surgery: two fatal cases. Forensic Sci Int Rep. 2023;7:100304. [FreeFullText]
- Thepsuwan J, Huang KG, Wilamarta M, Adlan AS, Manvelyan V, Lee CL. Principles of safe abdominal entry in laparoscopic gynecologic surgery. Gynecol Minim Invasive Ther. 2013;2(4):105-9. DOI: 1016/j.gmit.2013.07.003
- Gurusamy KS, Vaughan J, Davidson BR. Low pressure versus standard pressure pneumoperitoneum in laparoscopic cholecystectomy. Cochrane Database Syst Rev. 2014;2014(3):CD006930. [PubMed] DOI: 1002/14651858.CD006930.pub3