Kenneth Schmitt 1, Joseph Tafalla 2, Richard Wells 3, Joseph Rinehart 4
Author affiliations:
Women of childbearing age with surgically palliated congenital heart disease (CHD) have become increasingly common as survival rates of CHD improve. We describe a 33-year-old female with a history of Ebstein anomaly (EA), status post fenestrated lateral tunnel Fontan procedure, at gestational week 37 who presented for induction of labor and was found to have a fetal breech presentation. Cesarean section with general anesthesia was indicated after failure to progress in labor with epidural analgesia. As surgically treated EA has led to increased survivability, further discussion on the anesthetic management of this growing obstetric patient population was necessary.
Abbreviations: CO: cardiac output; CTPA: computed tomography pulmonary angiography; CHD: congenital heart disease; EA: Ebstein anomaly; LPA: left pulmonary artery; LT: lateral tunnel; PVR: pulmonary vascular resistance; RA: right atrium; SVC: superior vena cava; TTE: transthoracic echocardiogram; VR: venous return.
Keywords: Ebstein Anomaly; Congenital Heart Disease; Anesthesia; Fontan Procedure
Citation: Schmitt K, Tafalla J, Wells R, Rinehart J. Anesthetic management of a parturient with Ebstein anomaly palliated with Fontan procedure. Anaesth. pain intensive care 2025;29(2):341-344. DOI: 10.35975/apic.v29i2.2724
Received: July 18, 2023; Reviewed: December 03, 2023; Accepted: January 01, 2023
With continued improvement of survival rates, women of childbearing age with congenital heart disease (CHD) have become increasingly common.1 Caring for these patients requires a thorough understanding of the dramatically different physiologic demands of the patient’s specific anatomy and repair versus the physiologic changes of pregnancy. Even with marked improvements via surgical management, the anatomic and physiologic effects of Ebstein anomaly (EA) can persist and place peripartum patients at high risk of maternal and fetal complications.1 Prior studies have examined the management of pregnancy outcomes of patients with CHD,2,3 including those with unrepaired EA.4 However, there remains limited literature on the obstetric anesthetic management of surgically treated EA patients. We describe a parturient with a complex history of EA treated with Fontan surgery and complicated by failed induction of labor.
A 33-year-old gravida 2, para 0 female at 35 weeks gestation was admitted from high-risk obstetrics clinic for tachycardia of 140 beats/min while ambulating, and 110 beats/min while at rest. The patient was born with EA for which she underwent an initial bidirectional Glenn procedure at the age of 7 years and subsequent completion of fenestrated lateral tunnel Fontan surgery at the age of 8 years. Current cardiac anatomy consisted of a lateral tunnel intra-atrial baffle connecting the inferior vena cava through the right atrium to the right pulmonary artery. Medical history also included blood clots medicated with aspirin 81 mg daily, atrioventricular nodal reentrant tachycardia status post ablation, sinus node dysfunction status post dual chamber pacemaker placement, and a pacemaker generator change 12 years prior. For her current admission, sinus tachycardia resolved upon arrival, however, she continued to have shortness of breath while at rest. Oxygen saturation was 96% with no chest pain, extremity edema, or limb discoloration and labs were within normal limits of pregnancy. Electrocardiogram demonstrated normal sinus rhythm with incomplete right bundle branch block. A transthoracic echocardiogram (TTE) on admission showed a left ventricular ejection fraction of 55%. The results of a 9-month prior cardiac catheterization noted a pulmonary artery pressure of 14 mmHg. Exercise stress test performed 6 months back was normal. Pacemaker interrogation demonstrated no pacing with 3 months of battery life left and deemed sufficient to last throughout the course of pregnancy. Liver biopsies demonstrated Fontan-associated liver disease consisting of congestive hepatopathy and fibrosis with no evidence of liver cirrhosis. During her admission, she was started on metoprolol 75 mg daily, continued on aspirin 81 mg daily, and scheduled for induction of labor in 2 weeks’ time.
The patient was admitted to the hospital one day before her scheduled induction of labor at 37 weeks and was found to have a fetal breech presentation. Options were discussed and the decision to attempt an external cephalic version was agreed upon. After evaluation, she presented to the labor and delivery operating room where an epidural catheter was placed at L3-4 space with loss of resistance to saline technique. Lidocaine 2% was slowly administered 2-3 mL every 5 min. Due to supply constraints of lidocaine at our institution, ropivacaine was started; however, the procedure concluded quickly thereafter and only 1 mL of ropivacaine 0.5% was administered. Fentanyl IV was used to supplement the epidural for parts of the procedure, as the obstetric team noted it to be the most painful. The patient underwent a successful external cephalic version, but failed to progress in labor, receiving fentanyl 2 µg/mL with bupivacaine 0.1% and gradually titrating from 5 mL/h to 9 mL/h throughout her 18-hour induction of labor. A decision from the obstetric team was made to proceed with lower transverse cesarean section due to variable and late decelerations as well as failed induction of labor. Given the obstetric team’s clinical opinion of procedural urgency and the potentially increased risk of catastrophic high spinal, it was decided against a slowly titrated epidural in favor of general anesthesia. An arterial line was placed followed by induction with 50 mg propofol, 20 mg etomidate, and 100 mg of succinylcholine. Intubation was accomplished with direct laryngoscopy, and antibiotic coverage was provided with cefazolin and azithromycin as per institutional protocol.
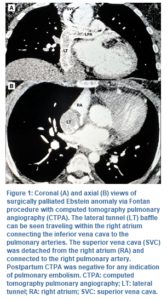
The delivery was without complications, with an estimated blood loss of 750 mL and total fluid administration of 1,000 mL crystalloid solution. A viable male baby was delivered weighing 2.92 kg and crown-ramp length (CRL) of 50.8 cm with an Apgar score of 7 and 9 at 1 and 5 min respectively. Postoperatively, oxytocin was used to manage postpartum bleeding. Brain natriuretic peptide was mildly elevated to 105 and resolved with one dose of furosemide and a postpartum echocardiogram demonstrated normal systolic function. On postoperative day 2, the patient was noted to have intermittent oxygen desaturations of 88% with concerns of pulmonary embolism versus the effects of Fontan circulation. A CT pulmonary angiograph was negative for pulmonary embolism (Figure 1), and oxygen saturation remained > 90% thereafter. The patient was discharged home 3 days postpartum in stable condition.
Ebstein’s anomaly (EA), a complex congenital malformation accounting for less than 1% of CHD, is characterized by downward displacement of the tricuspid valve, proximal right ventricular atrialization, and right atrial enlargement.5 Arrhythmias, heart failure, stroke, and paradoxical embolism are notable complications associated with EA. Ventricular tachycardia or a history of heart failure are also significantly linked with an elevated risk of sudden death.6, 7 Clinical significance and severity of right ventricular deformity are largely dependent on tricuspid valve displacement, and surgical intervention is often required for patients with severe congenital cardiac anomalies.8
For our case, a bidirectional Glenn procedure was first completed to separate the superior vena cava from the right atrium and connect it to the pulmonary artery. Fontan surgery was then accomplished with a fenestrated intra-atrial tunnel, directing inferior vena cava blood flow to the pulmonary artery (Figure 1). Once completed, blood flow bypasses the right ventricle and relies on right atrial pressures to direct passive blood flow to the pulmonary artery and lungs. Decreased venous return (VR) or increased pulmonary vascular resistance (PVR) will significantly decrease cardiac output (CO), given the reliance on passive VR in place of a right ventricle. Additionally, physiologic changes in pregnancy, including increased blood volume and CO, place additional strain on the single LV. This further increases the risk of heart failure and atrial tachyarrhythmia due to fluid retention and atrial distension.9 Coupled with a considerably increased risk of venous thromboembolism in the peripartum period, the significant risks of pregnancy are routinely counseled to women of childbearing age with such cardiac anomalies.10 In our case, the patient had been advised against pregnancy by her cardiologist but was not on contraceptives due to concerns of weight gain.
A primary goal for anesthetizing patients with such cardiac anomalies involves preserving CO with particular consideration of PVR. Prior testing including TTE as well as cardiopulmonary exercise stress testing provides critical information for assessing ventricular function and capability to tolerate the physiological changes of pregnancy. For our case, a recent 3rd-trimester TTE showed a normal systolic function and ejection fraction while a first trimester exercise stress test also came back normal. Epidural anesthesia with slow titration of anesthetics is preferred to minimize the risk of a sudden decrease in systemic vascular resistance.11 In our case, the patient was initially anesthetized with epidural anesthesia; however, later required general anesthesia due to late decelerations in fetal heart tracings and the procedural urgency. The combination of impaired VR from positive pressure ventilation and increased PVR from hypoxia and hypercarbia in a difficult intubation setting can lead to significantly decreased cardiac output. In cases such as ours where general anesthesia is necessary, it is essential to preserve forward-flowing circulation, including the maintenance of normal sinus rhythm and blood pressure while permitting increased heart rate within normal range. In order to achieve this, the patient was kept well-oxygenated, normothermic, and eucapnic throughout the procedure. An arterial line allowed regular measurement of pH and facilitated the ability to keep the patient non-acidotic. Protective ventilation measures can also be implemented including reduced tidal volumes and peak inspiratory pressures in order to further support CO and pulmonary blood flow.12
Postpartum, additional studies including brain natriuretic peptide and TTE play a role in examining volume status and cardiovascular function.1,13 Though there is no reported effect of EA on fertility, both EA and subsequent Fontan procedures are associated with an increased risk of abortion.14 Our patient was noted to have a first-trimester spontaneous abortion prior to her most recent pregnancy. Additionally, neonatal outcomes have been noted to be poor, including an increased risk of CHD in those born to mothers with EA.15 However, our patient delivered a viable male infant of normal size and weight for gestational age, and had a normal fetal echocardiogram 3 months back. This may partially be due to minimally reported oxygen desaturation throughout the course of pregnancy and reduced systemic venous mixing status post her Fontan procedure.15 Additionally, the anesthetic management of this patient may have had fewer complications given her normal left ventricular function in comparison to other palliated CHDs. The infant received two days of phototherapy in the newborn nursery, resolving mildly elevated bilirubin levels and was discharged home with mother on postoperative day 3.
EA is a complex form of CHD with surgical palliation allowing for increased quality of life and achievable childbearing age. Increased awareness of the differing parturient presentations, thorough anesthetic preparation, and a comprehensive physiologic understanding are crucial for a positive outcome.
4. Conflict of interest
The authors declare no conflicts of interest or financial interests to disclose. The study utilized the hospital resources only, and no external or industry funding was involved.
5. Ethics Committee Statement
Case reports are exempt from institutional committee review at our institution. Consent was obtained for the purpose of research and publication.
6. Authors’ contribution
KS: Assisted with data curation, literature review, manuscript revisions, figure conceptualization, and preparation of the manuscript for submission.
JT: Assisted with data curation, literature review, manuscript organization, and study logistics.
RW: Assisted with key content guidance, resource suggestions, data curation, and manuscript revisions.
JR: Established the importance of this case, provided an in-depth review of the manuscript, and assisted in the preparation of the manuscript for submission.
Author affiliations:
- Kenneth Schmitt, Department of Anesthesiology & Perioperative Care, University of California Irvine, CA, 92868, USA; Email: kennets@hs.uci.edu
- Joseph Tafalla, Department of Anesthesiology & Perioperative Care, University of California Irvine, CA, 92868, USA; Email: jtafalla@hs.uci.edu
- Richard Wells, Department of Anesthesiology & Perioperative Care, University of California Irvine, CA, 92868, USA; Email: wellsrt@hs.uci.edu
- Joseph Rinehart, Department of Anesthesiology & Perioperative Care, University of California Irvine, CA, 92868, USA; Email; jrinehar@hs.uci.edu
ABSTRACT
Women of childbearing age with surgically palliated congenital heart disease (CHD) have become increasingly common as survival rates of CHD improve. We describe a 33-year-old female with a history of Ebstein anomaly (EA), status post fenestrated lateral tunnel Fontan procedure, at gestational week 37 who presented for induction of labor and was found to have a fetal breech presentation. Cesarean section with general anesthesia was indicated after failure to progress in labor with epidural analgesia. As surgically treated EA has led to increased survivability, further discussion on the anesthetic management of this growing obstetric patient population was necessary.
Abbreviations: CO: cardiac output; CTPA: computed tomography pulmonary angiography; CHD: congenital heart disease; EA: Ebstein anomaly; LPA: left pulmonary artery; LT: lateral tunnel; PVR: pulmonary vascular resistance; RA: right atrium; SVC: superior vena cava; TTE: transthoracic echocardiogram; VR: venous return.
Keywords: Ebstein Anomaly; Congenital Heart Disease; Anesthesia; Fontan Procedure
Citation: Schmitt K, Tafalla J, Wells R, Rinehart J. Anesthetic management of a parturient with Ebstein anomaly palliated with Fontan procedure. Anaesth. pain intensive care 2025;29(2):341-344. DOI: 10.35975/apic.v29i2.2724
Received: July 18, 2023; Reviewed: December 03, 2023; Accepted: January 01, 2023
1. INTRODUCTION
With continued improvement of survival rates, women of childbearing age with congenital heart disease (CHD) have become increasingly common.1 Caring for these patients requires a thorough understanding of the dramatically different physiologic demands of the patient’s specific anatomy and repair versus the physiologic changes of pregnancy. Even with marked improvements via surgical management, the anatomic and physiologic effects of Ebstein anomaly (EA) can persist and place peripartum patients at high risk of maternal and fetal complications.1 Prior studies have examined the management of pregnancy outcomes of patients with CHD,2,3 including those with unrepaired EA.4 However, there remains limited literature on the obstetric anesthetic management of surgically treated EA patients. We describe a parturient with a complex history of EA treated with Fontan surgery and complicated by failed induction of labor.
2. CASE REPORT
A 33-year-old gravida 2, para 0 female at 35 weeks gestation was admitted from high-risk obstetrics clinic for tachycardia of 140 beats/min while ambulating, and 110 beats/min while at rest. The patient was born with EA for which she underwent an initial bidirectional Glenn procedure at the age of 7 years and subsequent completion of fenestrated lateral tunnel Fontan surgery at the age of 8 years. Current cardiac anatomy consisted of a lateral tunnel intra-atrial baffle connecting the inferior vena cava through the right atrium to the right pulmonary artery. Medical history also included blood clots medicated with aspirin 81 mg daily, atrioventricular nodal reentrant tachycardia status post ablation, sinus node dysfunction status post dual chamber pacemaker placement, and a pacemaker generator change 12 years prior. For her current admission, sinus tachycardia resolved upon arrival, however, she continued to have shortness of breath while at rest. Oxygen saturation was 96% with no chest pain, extremity edema, or limb discoloration and labs were within normal limits of pregnancy. Electrocardiogram demonstrated normal sinus rhythm with incomplete right bundle branch block. A transthoracic echocardiogram (TTE) on admission showed a left ventricular ejection fraction of 55%. The results of a 9-month prior cardiac catheterization noted a pulmonary artery pressure of 14 mmHg. Exercise stress test performed 6 months back was normal. Pacemaker interrogation demonstrated no pacing with 3 months of battery life left and deemed sufficient to last throughout the course of pregnancy. Liver biopsies demonstrated Fontan-associated liver disease consisting of congestive hepatopathy and fibrosis with no evidence of liver cirrhosis. During her admission, she was started on metoprolol 75 mg daily, continued on aspirin 81 mg daily, and scheduled for induction of labor in 2 weeks’ time.
The patient was admitted to the hospital one day before her scheduled induction of labor at 37 weeks and was found to have a fetal breech presentation. Options were discussed and the decision to attempt an external cephalic version was agreed upon. After evaluation, she presented to the labor and delivery operating room where an epidural catheter was placed at L3-4 space with loss of resistance to saline technique. Lidocaine 2% was slowly administered 2-3 mL every 5 min. Due to supply constraints of lidocaine at our institution, ropivacaine was started; however, the procedure concluded quickly thereafter and only 1 mL of ropivacaine 0.5% was administered. Fentanyl IV was used to supplement the epidural for parts of the procedure, as the obstetric team noted it to be the most painful. The patient underwent a successful external cephalic version, but failed to progress in labor, receiving fentanyl 2 µg/mL with bupivacaine 0.1% and gradually titrating from 5 mL/h to 9 mL/h throughout her 18-hour induction of labor. A decision from the obstetric team was made to proceed with lower transverse cesarean section due to variable and late decelerations as well as failed induction of labor. Given the obstetric team’s clinical opinion of procedural urgency and the potentially increased risk of catastrophic high spinal, it was decided against a slowly titrated epidural in favor of general anesthesia. An arterial line was placed followed by induction with 50 mg propofol, 20 mg etomidate, and 100 mg of succinylcholine. Intubation was accomplished with direct laryngoscopy, and antibiotic coverage was provided with cefazolin and azithromycin as per institutional protocol.
The delivery was without complications, with an estimated blood loss of 750 mL and total fluid administration of 1,000 mL crystalloid solution. A viable male baby was delivered weighing 2.92 kg and crown-ramp length (CRL) of 50.8 cm with an Apgar score of 7 and 9 at 1 and 5 min respectively. Postoperatively, oxytocin was used to manage postpartum bleeding. Brain natriuretic peptide was mildly elevated to 105 and resolved with one dose of furosemide and a postpartum echocardiogram demonstrated normal systolic function. On postoperative day 2, the patient was noted to have intermittent oxygen desaturations of 88% with concerns of pulmonary embolism versus the effects of Fontan circulation. A CT pulmonary angiograph was negative for pulmonary embolism (Figure 1), and oxygen saturation remained > 90% thereafter. The patient was discharged home 3 days postpartum in stable condition.
3. DISCUSSION
Ebstein’s anomaly (EA), a complex congenital malformation accounting for less than 1% of CHD, is characterized by downward displacement of the tricuspid valve, proximal right ventricular atrialization, and right atrial enlargement.5 Arrhythmias, heart failure, stroke, and paradoxical embolism are notable complications associated with EA. Ventricular tachycardia or a history of heart failure are also significantly linked with an elevated risk of sudden death.6, 7 Clinical significance and severity of right ventricular deformity are largely dependent on tricuspid valve displacement, and surgical intervention is often required for patients with severe congenital cardiac anomalies.8
For our case, a bidirectional Glenn procedure was first completed to separate the superior vena cava from the right atrium and connect it to the pulmonary artery. Fontan surgery was then accomplished with a fenestrated intra-atrial tunnel, directing inferior vena cava blood flow to the pulmonary artery (Figure 1). Once completed, blood flow bypasses the right ventricle and relies on right atrial pressures to direct passive blood flow to the pulmonary artery and lungs. Decreased venous return (VR) or increased pulmonary vascular resistance (PVR) will significantly decrease cardiac output (CO), given the reliance on passive VR in place of a right ventricle. Additionally, physiologic changes in pregnancy, including increased blood volume and CO, place additional strain on the single LV. This further increases the risk of heart failure and atrial tachyarrhythmia due to fluid retention and atrial distension.9 Coupled with a considerably increased risk of venous thromboembolism in the peripartum period, the significant risks of pregnancy are routinely counseled to women of childbearing age with such cardiac anomalies.10 In our case, the patient had been advised against pregnancy by her cardiologist but was not on contraceptives due to concerns of weight gain.

A primary goal for anesthetizing patients with such cardiac anomalies involves preserving CO with particular consideration of PVR. Prior testing including TTE as well as cardiopulmonary exercise stress testing provides critical information for assessing ventricular function and capability to tolerate the physiological changes of pregnancy. For our case, a recent 3rd-trimester TTE showed a normal systolic function and ejection fraction while a first trimester exercise stress test also came back normal. Epidural anesthesia with slow titration of anesthetics is preferred to minimize the risk of a sudden decrease in systemic vascular resistance.11 In our case, the patient was initially anesthetized with epidural anesthesia; however, later required general anesthesia due to late decelerations in fetal heart tracings and the procedural urgency. The combination of impaired VR from positive pressure ventilation and increased PVR from hypoxia and hypercarbia in a difficult intubation setting can lead to significantly decreased cardiac output. In cases such as ours where general anesthesia is necessary, it is essential to preserve forward-flowing circulation, including the maintenance of normal sinus rhythm and blood pressure while permitting increased heart rate within normal range. In order to achieve this, the patient was kept well-oxygenated, normothermic, and eucapnic throughout the procedure. An arterial line allowed regular measurement of pH and facilitated the ability to keep the patient non-acidotic. Protective ventilation measures can also be implemented including reduced tidal volumes and peak inspiratory pressures in order to further support CO and pulmonary blood flow.12
Postpartum, additional studies including brain natriuretic peptide and TTE play a role in examining volume status and cardiovascular function.1,13 Though there is no reported effect of EA on fertility, both EA and subsequent Fontan procedures are associated with an increased risk of abortion.14 Our patient was noted to have a first-trimester spontaneous abortion prior to her most recent pregnancy. Additionally, neonatal outcomes have been noted to be poor, including an increased risk of CHD in those born to mothers with EA.15 However, our patient delivered a viable male infant of normal size and weight for gestational age, and had a normal fetal echocardiogram 3 months back. This may partially be due to minimally reported oxygen desaturation throughout the course of pregnancy and reduced systemic venous mixing status post her Fontan procedure.15 Additionally, the anesthetic management of this patient may have had fewer complications given her normal left ventricular function in comparison to other palliated CHDs. The infant received two days of phototherapy in the newborn nursery, resolving mildly elevated bilirubin levels and was discharged home with mother on postoperative day 3.
EA is a complex form of CHD with surgical palliation allowing for increased quality of life and achievable childbearing age. Increased awareness of the differing parturient presentations, thorough anesthetic preparation, and a comprehensive physiologic understanding are crucial for a positive outcome.
4. Conflict of interest
The authors declare no conflicts of interest or financial interests to disclose. The study utilized the hospital resources only, and no external or industry funding was involved.
5. Ethics Committee Statement
Case reports are exempt from institutional committee review at our institution. Consent was obtained for the purpose of research and publication.
6. Authors’ contribution
KS: Assisted with data curation, literature review, manuscript revisions, figure conceptualization, and preparation of the manuscript for submission.
JT: Assisted with data curation, literature review, manuscript organization, and study logistics.
RW: Assisted with key content guidance, resource suggestions, data curation, and manuscript revisions.
JR: Established the importance of this case, provided an in-depth review of the manuscript, and assisted in the preparation of the manuscript for submission.
7. REFERENCES
- Correction to: 2018 AHA/ACC Guideline for the Management of Adults With Congenital Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019 Apr 2;139(14):e833-e834. [PubMed] DOI: 1161/CIR.0000000000000683
- Hayward RM, Foster E, Tseng ZH. Maternal and Fetal Outcomes of Admission for Delivery in Women With Congenital Heart Disease. JAMA Cardiol. 2017;2(6):664-671. [PubMed] DOI: 1001/jamacardio.2017.0283
- Choi EY, Kim ES, Kim JY, Song MK, Kim SH, Noh CI. Pregnancy outcomes in patients with structural heart disease: a single center experience.Cardiovasc Diagn Ther. 2021;11(1):81-90. [PubMed] DOI: 21037/cdt-20-786
- Sharma N, Lalnunnem TJ, Nandwani M, Santa SA, Synrang BW. Ebstein Anomaly with Pregnancy: A Rare Case.J Reprod Infertil. 2018;19(2):119-122. [PubMed]
- Paranon S, Acar P. Ebstein's anomaly of the tricuspid valve: from fetus to adult: congenital heart disease.Heart. 2008;94(2):237-243. [PubMed] DOI: 1136/hrt.2006.105262
- Gentles TL, Calder AL, Clarkson PM, Neutze JM. Predictors of long-term survival with Ebstein's anomaly of the tricuspid valve.Am J Cardiol. 1992;69(4):377-381. [PubMed] DOI: 1016/0002-9149(92)90237-s
- Attenhofer Jost CH, Tan NY, Hassan A, Vargas ER, Hodge DO, Dearani JA, et al. Sudden death in patients with Ebstein anomaly.Eur Heart J. 2018;39(21):1970-1977a. [PubMed] DOI: 1093/eurheartj/ehx794
- Bove EL, Hirsch JC, Ohye RG, Devaney EJ. How I manage neonatal Ebstein's anomaly.Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu. 2009;63-65. [PubMed] DOI: 1053/j.pcsu.2009.01.023
- McCabe M, An N, Aboulhosn J, Schwarzenberger J, Canobbio M, Vallera C, et al. Anesthetic management for the peripartum care of women with Fontan physiology.Int J Obstet Anesth. 2021;48:103210. [PubMed] DOI: 1016/j.ijoa.2021.103210
- Earing MG, Webb GD. Congenital heart disease and pregnancy: maternal and fetal risks.Clin Perinatol. 2005;32(4):913-ix. [PubMed] DOI: 1016/j.clp.2005.09.004
- Hosking MP, Beynen FM. The modified Fontan procedure: physiology and anesthetic implications.J Cardiothorac Vasc Anesth. 1992;6(4):465-475. [PubMed] DOI: 1016/1053-0770(92)90017-2
- Al-Eyadhy A. Mechanical ventilation strategy following Glenn and Fontan surgeries: On going challenge!J Saudi Heart Assoc. 2009;21(3):153-157. [PubMed] DOI: 1016/j.jsha.2009.06.005
- Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration with the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons.J Am Coll Cardiol. 2008;52(23):e143-e263. [PubMed] DOI: 1016/j.jacc.2008.10.001
- Nataloni M, Mocchegiani R. Ebstein's anomaly and pregnancy: a case report.Ital Heart J. 2004;5(9):707-710. [PubMed]
- Suriya JY, Raj A, Pillai AA, Satheesh S, Plakkal N, Kundra P, Keepanasseril A, et al. Ebstein's anomaly during pregnancy: experience from a tertiary care centre - a case series and review of literature.J Obstet Gynaecol. 2022;42(4):594-596. [PubMed] DOI: 1080/01443615.2021.1932777