Mohamed Zedan 1, Mohamed Elwasseef 2, Ehab Hanafy Shaker 2, Doaa Abdeltawab Mohamed Turki 2, Mahmoud A. Kamel 2
Author Affiliations:
Background & objectives: The anesthetists have been trying various adjuvants with local anesthetic agents for spinal anesthesia to prolong the duration of analgesia and to reduce the associated side effects. This study was aimed to evaluate the effect of dexmedetomidine versus midazolam when added to the intrathecal bupivacaine.
Methods: Seventy-five adult patients, 20 to 70 y of age, scheduled for orthopedic cancer procedures under the spinal anesthesia, were enrolled in this research trial. Selected patients were randomly divided into three equal groups; Group D (Dexmed Group): 25 patients received spinal anesthesia with 0.5% bupivacaine 3.5 mL plus dexmedetomidine 5 μg in saline 0.5 mL; Group M (Midazolam Group) 25 patients received 0.5% bupivacaine 3.5 mL plus midazolam 2 mg in 0.5 mL normal saline, and the third group Group C (Control Group) comprising of 25 patients received 0.5% bupivacaine 3.5 mL plus 0.5 mL normal saline. The primary outcome of this study was duration of the sensory block. Secondary outcomes were duration of the motor block.
Results: We employed two segments of time. The median duration of the dexmedetomidine group was 132 min, which is statistically significant compared to the midazolam group (119 min) and the control group (98 min). The motor block was also significantly prolonged in the Dexmed Group compared to the midazolam group and the control group. However, dexmedetomidine and midazolam groups showed significant hypotension compared to the control group.
Conclusion: Dexmedetomidine has a longer sensory and motor block effect than midazolam, when added to the spinal bupivacaine; both dexmedetomidine and midazolam groups have longer duration than the control group. Dexmedetomidine can be recommended for prolonged orthopedic cancer surgeries, due to its prolonged postoperative analgesia despite the associated hypotension.
Abbreviations: GABA: gamma-aminobutyric acid. gamma-aminobutyric acid, MAP: mean arterial pressure.
Keywords: Intrathecal Dexmedetomidine; Intrathecal Midazolam; Hyperbaric Bupivacaine; Spinal Anesthesia, Hypotension
Citation: Zedan M, Elwasseef M, Shaker EH, Mohamed Turki DA, Kamel MA. Comparing intrathecal dexmedetomidine versus midazolam in orthopedic cancer surgeries: a prospective randomized controlled trial. Anaesth. pain intensive care 2025;29(2):185-193. DOI: 10.35975/apic.v29i2.2702
Received: September 24, 2024; Reviewed: October 26, 2024; Accepted: January 25, 2025
Globally, spinal anesthesia has been the standard procedure for lower extremities and abdominal surgery. It is a very dependable and acceptable approach for lower limb surgeries.1 Significant postoperative pain is often experienced by patients undergoing orthopedic surgeries in the lower limbs, requiring the use of parenteral or neuraxial opioids for sufficient analgesia during the postoperative phase. Local anesthetic drugs (intrathecal 0.5% bupivacaine) are regularly used for neuraxial blocking. Its duration of action is considerably shorter when taken alone, and early analgesic interference is necessary throughout the postoperative phase.2
Intrathecal local anesthetics have been used with a number of additives to increase the duration of postoperative analgesia and enhance intraoperative anesthesia. They offer benefits because they reduce the amount of local anesthetic and provide long-term postoperative analgesia while decreasing the risk of central nervous system inhibition, motor symptoms, or hypotension. But their adverse effects, such as urine retention, respiratory depression, nausea, vomiting, itching, and drowsiness, restrict their use as adjuvants. Trials continue to be conducted to investigate the favorable and negative aspects of one adjuvant over the other.3
Dexmedetomidine is a very specific alpha-2-adrenoreceptor agonist along with significant impacts regarding the central nervous system, reducing the tone of the sympathetic system. When intrathecal anesthetics are combined with dexmedetomidine, the analgesic effect of the dexmedetomidine can be demonstrated by the suppression of the C-fiber transmitter release plus hyperpolarization to the postsynaptic dorsal horn neurons, which explains prolonged duration of spinal block. It has been skillfully employed via a parenteral route for the management and shivering prevention after spinal anesthesia without any significant adverse effects in multiple studies.4
It has been demonstrated that intrathecal midazolam has analgesic qualities and enhances the effects of subarachnoid local anesthetic. The process by which midazolam induces analgesic effect has been studied in various recent trials. It functions via gamma-aminobutyric acid (GABA) receptors located in the spinal cord's dorsal horn. The lamina II of the dorsal horn ganglia, which is important in dealing with nociceptive and thermoceptive stimuli, has the highest concentration of these receptors. It may also have a central antinociceptive action via spinal opioid receptor activation.5
Although there are previous studies investigating intrathecal dexmedetomidine versus midazolam as adjuvant to bupivacaine, to our knowledge, we did not find any study comparing both drugs in orthopedic cancer surgeries.
This study aimed to compare adding dexmedetomidine versus midazolam as an additive to bupivacaine with spinal anesthesia in orthopedic cancer surgeries with regard to sensory block, motor block, and adverse effects.
A controlled, randomized, double-blinded trial took place in the National Cancer Institute Hospital, Cairo University, Egypt, after the consent of the Institutional Review Board (IRB number AP 210330102). Clinicaltrials.gov provided the study's ID, NCT06315634, upon registration. Following the Consolidated Standards of Reporting Trials (CONSORT), written informed consent was gathered from each patient.
Adult patients 20 years to 70 years old of both genders (ASA I-III) arranged for orthopedic cancer operations under spinal anesthesia were enrolled in the trial.
Patients with compromised mental status, individuals suffering from coagulopathy, patients have a history of local anesthetics or dexmedetomidine allergy, patients have severe valvular stenosis, psychiatric conditions, or patients with histories of chronic use or abuse of narcotics or drug abuse were excluded from the study.
Three groups of patients were randomly assigned using computer-generated random numbers that were kept in sealed envelopes.• Group D: to receive intrathecal hyperbaric bupivacaine 0.5% (17.5 mg) 3.5 mL + 5 μg of dexmedetomidine in 0.5 mL saline.
2.1. Spinal anesthesia technique
Using a 25-gauge needle and 2 mL of 2% lignocaine, local infiltration was done to a sitting patient under aseptic conditions. Subsequently, with a midline approach and a 25-gauge Quincke spinal needle (Spinocan, B-Braun Melsungen AG, Melsungen, Germany), spinal anesthesia was delivered at the L4–L5 level. After appearance of CSF, 4 mL of the prepared solution was injected according to the selected group. Patients were then placed into supine positions. The sensory block was measured using a short bevel, 25G needle in the pin prick test, and the motor assessment was conducted using the Bromage scale.6
Lactated ringer solution was infused at a rate of 4 mL/kg/h. Hypotension was managed with 5 mg increments of ephedrine to keep mean arterial pressure (MAP) above 70 mmHg. If necessary, 0.5 mg of atropine was given to treat bradycardia, defined as a heart rate below 50 beats per min.
Duration of the sensory block, the primary outcome, was defined as the time period from completion of intrathecal drug administration, to 2-segment regression of sensory block. Secondary outcomes were set as: (i) Time to onset of sensory block (the period between the application of spinal anesthesia and the total loss of skin sensation to the pinprick test up to T10 level); (ii) Time to onset of motor block (described as the duration from the administration of spinal anesthesia to the extended modified Bromage scale of one, (iii) Motor block duration was specified as the duration from the delivery of spinal anesthesia to the extended modified Bromage scale of zero, (iv) perioperative HR and MAP, (v) shivering; the frequency and intensity of shivering using the Crossley and Mahajan scale,7 patient was regarded as shivering when scoring was 2 or more. (vi) Vomiting, (vii) Degree of sedation utilizing the Ramsay sedation scale.8 Patients were considered sedated when their score was 4 or greater.
2.2. Sample size calculation
A previous study by Aloka Samantaray,9 indicated the sensory block mean duration by dexmedetomidine was 286 min, for midazolam it was 236.9 min, and for the control group it was 212.7. To detect a real difference in means across groups with a power of 80% and a significance of 5% (two-sided), an overall sample size of 66 patients was required; i.e., 22 patients in all three groups. To allow for potential drop-outs; the overall sample size was 75 patients (25 patients in each group).
2.3. Statistical analysis
The data was tabulated using Microsoft Office Excel 2010 for Windows. Analysis was carried out using the IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. The chi-square test was employed to analyze categorical variables, which were reported as frequencies (percentages).
The normality of the data distribution was established using the Shapiro-Wilk test. A repeated measures general linear model analysis of variance (ANOVA) was used to assess normally distributed data, which was subsequently provided as means and standard deviations (SD). Whenever data was determined not to be normally distributed, the Kruskal-Wallis test was employed, and the findings were displayed as medians and interquartile ranges (IQR). The Bonferroni test was carried out as a post-hoc test. A P < 0.05 was considered significant.
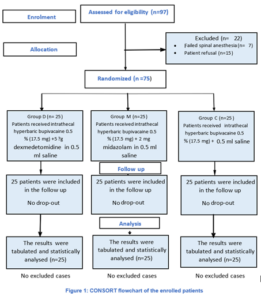
In the present trial 97 patients were examined for eligibility, 7 patients failed spinal anesthesia, and 15 patients refused to participate in the trial. Seventy-five adult patients were analyzed statistically (Figure 1).
The median age of enrolled patients was 44 years (IQR = 30.5–58.5). Regarding (ASA) physical condition classification, 57% of patients were ASA class I, 28% were class II, and just 14.7% were class III.
The median duration of surgery was 163 min; the median time to achieve sensory level to T10 level was 4 min; and the median time to reach motor block level was 8 minas shown in Table 1. The comparison of the three study groups, was based on demographics showed no significant differences (P > 0.05) (Table 2).
The median duration of motor blockade was 250 min for Group D patients, 153 min for Group M patients, and 125 min for Group C patients, and this difference was statistically significant (P = 0.026). Post-hoc tests found significant differences among all of the comparisons between pairs.
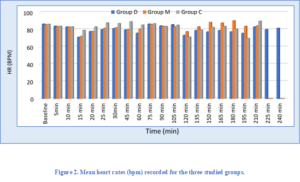
The two-segment regression time was 132 min for Group D patients, 119 min in Group M patients, and 98 min in Group C patients, and this difference was statistically significant (P < 0.001). Post-hoc tests found significant differences among all of the pairwise comparisons. There were no significant differences in the median time to reach sensory level T10 or the median time to achieve motor block level among the studied groups (Table 3). There was a significant difference in the recorded heart rates among the groups in the time period between 15 min to 60 min following the start of the surgery. Post-hoc tests showed that heart rate was considerably higher in Group C, compared to Group D and M (Figure 2).
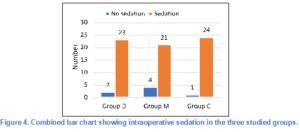
36% of patients in Group D experienced intraoperative bradycardia, 20% in Group M, and only 8% in Group C, and this difference was statistically significant (P = 0.0241) (Figure 3).
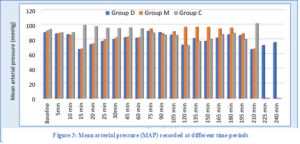
There was a significant difference in the recorded MAP among the three groups from 10 to 75 min and between 120 to 150 min after the start of the operation. MAP was significantly higher in Group C in comparison with the other two groups in the period 10 to 75 min and higher in Group M when compared to the other two groups in the period between 120 to 150 min (Figure 4).
Regarding the comparison of the recorded intraoperative hypotension at different time points, there was a significant difference in the recorded episodes of hypotension among the three groups in the time period between 15 min and 30 min and between 120 and 135 min after the start of the intrathecal injection. Post-hoc tests revealed that Groups D and M had considerably greater frequencies of hypotension episodes than Group C.
There were no significant differences among the three groups regarding SpO2 at any particular moment.
Ramsay’s sedation scores at different time points were not significantly difference among the three groups at any particular period (Table 4).
Vomiting was reported in 3 patients in Group D, 2 patients in Group M, and in one patient in Group C (Table 5). Shivering Grade II was documented in three patients in Group D, two patients in Group M, and two patients in Group C. Shivering in Grade III was noticed in three subjects in Group C. There were no significant differences between the three groups.
The most important finding of our study is both dexmedetomidine and midazolam prolonged both sensory and motor duration compared to control group with added privilege to dexmedetomidine. On the other hand, hypotension represents a minor complain in dexmedetomidine group compared to the other groups.
Spinal anesthesia is typically utilized for lower limb orthopedic surgery, including orthopedic cancer operations, since spinal anesthesia is a dependable and effective method with the advantage of postoperative analgesia and limited adverse effects. Adjuvants were used in conjunction with hyperbaric bupivacaine to extend the period of the sensory block, enhance postoperative analgesia, and increase the block's quality; however, these benefits came with a risk of hemodynamic instability, nausea, vomiting, and drowsiness.
Both midazolam and dexmedetomidine are fairly Recent entries to the list of adjuvants employed in spinal anesthesia and may function synergistically with intrathecal bupivacaine to lengthen the spinal anesthesia duration and for postoperative analgesia. When administered intrathecally, the two medications' mechanisms of action for producing antinociception are different.
Dexmedetomidine, an imidazoline molecule, is a D-isomer of dexmedetomidine, which is pharmacologically potent and displays specific α-2 adrenoceptor agonistic activity. Local anesthetics' motor and sensory block is extended by intrathecal α2-adrenoceptor agonists, which bind to postsynaptic dorsal horn neurons and presynaptic C-fibers. Depression of the release of C-fiber transmitters (substance P and glutamate) and postsynaptic dorsal horn neurons hyperpolarization cause this analgesic action.10
Al-Ghanem et al. performed a comparison between the effects of intrathecal 10 mg isobaric bupivacaine with 5μg dexmedetomidine and 25 mg fentanyl for vaginal hysterectomy. They found that the addition of 5 μg dexmedetomidine caused longer duration of motor and sensory block and fewer adverse reactions, such as bradycardia, hypotension, and pruritus, when compared to 25 μg fentanyl.11
In urological procedures, Al-Mustafa et al. evaluated the effects of 5 g and 10 g of dexmedetomidine with hyperbaric bupivacaine and showed that dexmedetomidine extends the time frame of spinal anesthesia in a dose-related manner.12
Kanazi GE et al. evaluated the impact of a low dose of dexmedetomidine (3 μg) compared to clonidine (30μg) when used in combination with bupivacaine intrathecally in transurethral resection of the prostate and bladder tumors. According to their findings, intrathecal dexmedetomidine had a quicker motor block onset and a longer duration of both motor and sensory block while maintaining hemodynamic stability and causing no sedation.13
The α-2 adrenergic agonist effects in the brain and spinal cord attenuate the sympathetic tone and decrease the catecholamines, thus avoiding vasoconstriction and increasing the threshold of shivering, so dexmedetomidine has anti-shivering features, as noticed by Usta B et al., who examined the impact of parenteral infusion of dexmedetomidine in the perioperative period compared to saline infusion during elective surgeries performed under spinal anesthesia and found that dexmedetomidine decreases perioperative incidence and severity of shivering.14
Hala et al. compared 10 μg and 15 μg of dexmedetomidine added to hyperbaric bupivacaine, and they determined that dexmedetomidine prolonged sensory and motor blockade and decreased postoperative pain and analgesic consumption when compared with the control group, with no side-effects between the three groups except for sedation with 15 μg.15
Local anesthetic drugs function by inhibiting sodium channels. The synergy between α2-adrenoceptor agonists and local anesthetics may prolong their action, whereas the attachment of α2-adrenoceptor agonists to motor neurons in the dorsal horn could extend the motor block of spinal anesthetics.5 Yegin et al. found that following perianal surgery, 2 mg of intrathecal midazolam produced mild drowsiness and had a longer postoperative analgesic effect than the control group. Midazolam, when given intrathecally, may cause exposure to analgesic systems influenced by GABA.16
Kohno et al. stated that the analgesic property of intrathecal midazolam is produced by an influence on the GABAergic spread in substantia gelatinosa neurons in spinal cord slices of adult rats.17 Intrathecal Midazolam plays a role in the outflow of endogenous opioids. Acting on spinal delta receptors, the antinociceptive impacts of morphine-like compounds are enhanced when intrathecal Midazolam is administered.17
Midazolam inhibits excitatory synaptic transmission by acting on the gamma aminobutyric acid type A/benzodiazepine receptor in interneurons, resulting in a decrease in the excitability of spinal dorsal horn neurons. Precautions must be made when administering intrathecal midazolam in clinical settings in humans because several studies have yielded inconsistent findings on the drug's possible neurotoxicity in animals. Few animal experiments have discovered histological proof of neurotoxicity in rats and rabbits following the administration of intrathecal midazolam.18, 19, 20 Other histological experiments in animals demonstrated that intrathecal midazolam does not generate notable alterations in the spinal cord.21, 22
Borg and Krijnen additionally stated that the continuous intrathecal delivery of midazolam to patients with resistant chronic benign pain produced quick and almost complete relief of pain with practically no adverse effects, and patients exhibited no tolerance to the midazolam analgesic effect.23
Shrivastav, R. et al. compared 5 μg dexmedetomidine with 1 mg of midazolam and the control group in any surgery done with spinal anesthesia. He observed that the beginning of the sensory block of dexmedetomidine is statistically faster than midazolam and control groups, while the midazolam group is statistically faster than the control group. The duration of sensory blockade by pin brick, dexmedetomidine, is statistically longer than midazolam and to control groups. The three groups had similar hemodynamics, but the dexmedetomidine group had more bradycardia.24
Aloka Samantaray et al. evaluated adding dexmedetomidine 5 μg vs. midazolam 1 mg to intrathecal bupivacaine in endourological procedures. The sensory block duration was observed to be statistically longer in the dexmedetomidine group than midazolam and the control group. Dexmedetomidine induced sedation in the first 30 min, with no nausea or vomiting. Dexmedetomidine generates hypotension in 35% of patients and bradycardia in 25% of patients, although the difference in hypotension or bradycardia was not significant.9
Al-Arnous MO, et al. compared intrathecal dexmedetomidine 5 μg and midazolam 2 mg with a control group in lower limb orthopedic procedures. While neither dexmedetomidine nor midazolam reduced blood pressure, they did lengthen sensory or motor duration; however, the dexmedetomidine group suffered more bradycardia than the midazolam group.25
In our current study, we examined the addition of 5 μg Dexmedetomidine against 2 mg midazolam to 0.5% hyperbaric bupivacaine in spinal anesthesia for lower limb orthopedic cancer procedures. The current study's primary outcome was the duration of sensory black.
Regarding the duration of sensory block, we used two segments regression time: the median duration of sensory block for the dexmedetomidine group is 132 min, which is statistically longer than midazolam and control groups; the median duration of sensory block for the midazolam group is 119 min, which is statistically longer than the control group, in which the median duration of sensory block is 98 min. The results we obtained are in line with those of Al-Arnous MO et al.25 and Shrivastav, R. et al.,24 but not with those of Aloka Samantaray et al.,9 who used only 1 mg of midazolam in contrast to our study, which used 2 mg. This difference may have contributed to our research's longer duration of sensory blockage.
The motor block for Group Dexmedetomidine has a median duration of 250 min. Which is significantly longer than midazolam and control groups; the median duration of motor block for the midazolam group is 153 min. Which is significantly greater than the control group, in which the median duration for motor block is 125 min.
There was not been a statistically significant variance in sensory or motor block onset among the three groups. Regarding the secondary outcome, there was no statistically significant variation in shivering or vomiting between the three groups. Most studies showed neither shivering nor vomiting.
For bradycardia, 36% of patients in the dexmedetomidine group had at least one episode of bradycardia, while only 20% of patients in the midazolam group had bradycardia and 8% of patients in the control group had bradycardia, which was statistically significant across the three groups.
In terms of hypotension, the dexmedetomidine group and the midazolam group were statistically significantly different from the control group. Unlike Aloka Samantaray et al.,9 Shrivastav, R. et al.,24 and Mahmoud Omar Al-Arnous.25 The sedation was monitored using the Ramsay sedation score. 4 patients scored III in Ramsay sedation scores in the midazolam group, whereas only 2 patients in the dexmedetomidine group were sedated and one patient in the control group; however, this difference did not appear significant statistically.
Relative to shivering and vomiting, there were no statistically significant variations across the three groups under assessment.
Unfortunately, we were not able to perform serum level of both drugs to assess the degree of systemic absorption, which represent limitation to this study. We recommend this in future researches as it will make a solid conclusion about the exact mechanism of action of both drugs as adjuvant to bupivacaine in spinal anesthesia for prolongation of both sensory and motor duration.
When used as additives in spinal anesthesia, dexmedetomidine has longer sensory and motor block than midazolam, and both dexmedetomidine and midazolam were longer in duration than the control group, so we recommend that dexmedetomidine is more efficient for lengthy operations like orthopedic malignant surgeries and can be utilized for prolongation of postoperative analgesia despite the side effects like hypotension and bradycardia, which can be managed by fluids, vasopressors, and anticholinergic drugs.
7. Funding
No grants from public, commercial, or not-for-profit funding organizations were given to the writers.
8. Availability of data
Data can be obtained from the authors upon reasonable inquiry after approval of the National Cancer Institute, Cairo University, Egypt.
9. Conflict of interest
The authors declare that there is no conflict of interest with any financial entity regarding the material presented in the work.
10. Consent for publication
The writers take responsibility for sharing this content. This transfer of publication rights includes a non-exclusive license to reproduce and distribute the article, including copies, translations, photographic reproductions, microform, digital format (offline, online), or any other similar kind of replication.
11. Ethics approval
On March 14, 2021, the Institutional Review Board (IRB number AP2103-301-02) approved the study, which was then carried out at the National Cancer Institute Hospital.
12.Clinical trial registration
The study was registered with Clinicaltrials.gov with ID No. NCT06315634.
13. Authors contribution
MZ, MK: Conception of the idea
MK, EH, ME, DT and MZ: Design of the study, analysis of the data, writing the manuscript. data collection; revised, and approved the final manuscript.
Author Affiliations:
- Mohamed Zedan, Department of Anesthesiology, Surgical ICU & Pain Management, Armed Forces College of Medicine, Cairo, Egypt; Email: mohammedzedan999@gmail.com
- Mohamed Elwasseef, Department of Anesthesiology, Surgical ICU and & Pain Management, National Cancer Institute, Cairo University, Cairo, Egypt; Email: wasseef@hotmail.com
- Ehab Hanafy Shaker, Department of Anesthesiology, Surgical ICU and & Pain Management, National Cancer Institute, Cairo University, Cairo, Egypt; Email: Ehabhemafy2006@yahoo.com
- Doaa Abdeltawab Mohamed Turki, Department of Anesthesiology, Surgical ICU and & Pain Management, National Cancer Institute, Cairo University, Egypt; Email: doaa.turki@nci.cu.edu.eg
- Mahmoud A. Kamel, Department of Anesthesiology, Surgical ICU and & Pain Management, National Cancer Institute, Cairo University, Cairo, Egypt; Email: mkamel_76@hotmail.com
ABSTRACT
Background & objectives: The anesthetists have been trying various adjuvants with local anesthetic agents for spinal anesthesia to prolong the duration of analgesia and to reduce the associated side effects. This study was aimed to evaluate the effect of dexmedetomidine versus midazolam when added to the intrathecal bupivacaine.
Methods: Seventy-five adult patients, 20 to 70 y of age, scheduled for orthopedic cancer procedures under the spinal anesthesia, were enrolled in this research trial. Selected patients were randomly divided into three equal groups; Group D (Dexmed Group): 25 patients received spinal anesthesia with 0.5% bupivacaine 3.5 mL plus dexmedetomidine 5 μg in saline 0.5 mL; Group M (Midazolam Group) 25 patients received 0.5% bupivacaine 3.5 mL plus midazolam 2 mg in 0.5 mL normal saline, and the third group Group C (Control Group) comprising of 25 patients received 0.5% bupivacaine 3.5 mL plus 0.5 mL normal saline. The primary outcome of this study was duration of the sensory block. Secondary outcomes were duration of the motor block.
Results: We employed two segments of time. The median duration of the dexmedetomidine group was 132 min, which is statistically significant compared to the midazolam group (119 min) and the control group (98 min). The motor block was also significantly prolonged in the Dexmed Group compared to the midazolam group and the control group. However, dexmedetomidine and midazolam groups showed significant hypotension compared to the control group.
Conclusion: Dexmedetomidine has a longer sensory and motor block effect than midazolam, when added to the spinal bupivacaine; both dexmedetomidine and midazolam groups have longer duration than the control group. Dexmedetomidine can be recommended for prolonged orthopedic cancer surgeries, due to its prolonged postoperative analgesia despite the associated hypotension.
Abbreviations: GABA: gamma-aminobutyric acid. gamma-aminobutyric acid, MAP: mean arterial pressure.
Keywords: Intrathecal Dexmedetomidine; Intrathecal Midazolam; Hyperbaric Bupivacaine; Spinal Anesthesia, Hypotension
Citation: Zedan M, Elwasseef M, Shaker EH, Mohamed Turki DA, Kamel MA. Comparing intrathecal dexmedetomidine versus midazolam in orthopedic cancer surgeries: a prospective randomized controlled trial. Anaesth. pain intensive care 2025;29(2):185-193. DOI: 10.35975/apic.v29i2.2702
Received: September 24, 2024; Reviewed: October 26, 2024; Accepted: January 25, 2025
1. INTRODUCTION
Globally, spinal anesthesia has been the standard procedure for lower extremities and abdominal surgery. It is a very dependable and acceptable approach for lower limb surgeries.1 Significant postoperative pain is often experienced by patients undergoing orthopedic surgeries in the lower limbs, requiring the use of parenteral or neuraxial opioids for sufficient analgesia during the postoperative phase. Local anesthetic drugs (intrathecal 0.5% bupivacaine) are regularly used for neuraxial blocking. Its duration of action is considerably shorter when taken alone, and early analgesic interference is necessary throughout the postoperative phase.2
Intrathecal local anesthetics have been used with a number of additives to increase the duration of postoperative analgesia and enhance intraoperative anesthesia. They offer benefits because they reduce the amount of local anesthetic and provide long-term postoperative analgesia while decreasing the risk of central nervous system inhibition, motor symptoms, or hypotension. But their adverse effects, such as urine retention, respiratory depression, nausea, vomiting, itching, and drowsiness, restrict their use as adjuvants. Trials continue to be conducted to investigate the favorable and negative aspects of one adjuvant over the other.3
Dexmedetomidine is a very specific alpha-2-adrenoreceptor agonist along with significant impacts regarding the central nervous system, reducing the tone of the sympathetic system. When intrathecal anesthetics are combined with dexmedetomidine, the analgesic effect of the dexmedetomidine can be demonstrated by the suppression of the C-fiber transmitter release plus hyperpolarization to the postsynaptic dorsal horn neurons, which explains prolonged duration of spinal block. It has been skillfully employed via a parenteral route for the management and shivering prevention after spinal anesthesia without any significant adverse effects in multiple studies.4
It has been demonstrated that intrathecal midazolam has analgesic qualities and enhances the effects of subarachnoid local anesthetic. The process by which midazolam induces analgesic effect has been studied in various recent trials. It functions via gamma-aminobutyric acid (GABA) receptors located in the spinal cord's dorsal horn. The lamina II of the dorsal horn ganglia, which is important in dealing with nociceptive and thermoceptive stimuli, has the highest concentration of these receptors. It may also have a central antinociceptive action via spinal opioid receptor activation.5
| Table 1: Basic features of the studied patients (N=75). | |
| Parameters | Studied cases |
| Age (y) | 44 (30.5–58.5) |
| ASA physical status | |
| · I | 43 (57.3%) |
| · II | 21 (28%) |
| · III | 11 (14.7%) |
| Type of operation | |
| · Extended intralesional curettage with cementing | 13 (17.3%) |
| · Tumor resection and vascularity grafting | 16 (21.3%) |
| · Above knee amputation | 17 (22.6%) |
| · Below knee amputation | 15 (20%) |
| · Wide margin resection and recycling | 14 (18.6%) |
| Duration of surgery (min) | 163 (112–197.5) |
| IV fluids (mL) | 2000 (1825–2350) |
| Time to reach sensory level T10 (min) | 4 (2–5) |
| Time to reach motor block level Bromage 1 (min) | 8 (6.5–9) |
| Duration of motor blockade (min) | 172 (125–215) |
| Two segment regression time (min) | 116 (104–128) |
| Values are expressed as Median (IQR), or Number (%) | |
Although there are previous studies investigating intrathecal dexmedetomidine versus midazolam as adjuvant to bupivacaine, to our knowledge, we did not find any study comparing both drugs in orthopedic cancer surgeries.
This study aimed to compare adding dexmedetomidine versus midazolam as an additive to bupivacaine with spinal anesthesia in orthopedic cancer surgeries with regard to sensory block, motor block, and adverse effects.
2. METHODOLOGY
A controlled, randomized, double-blinded trial took place in the National Cancer Institute Hospital, Cairo University, Egypt, after the consent of the Institutional Review Board (IRB number AP 210330102). Clinicaltrials.gov provided the study's ID, NCT06315634, upon registration. Following the Consolidated Standards of Reporting Trials (CONSORT), written informed consent was gathered from each patient.
Adult patients 20 years to 70 years old of both genders (ASA I-III) arranged for orthopedic cancer operations under spinal anesthesia were enrolled in the trial.

Patients with compromised mental status, individuals suffering from coagulopathy, patients have a history of local anesthetics or dexmedetomidine allergy, patients have severe valvular stenosis, psychiatric conditions, or patients with histories of chronic use or abuse of narcotics or drug abuse were excluded from the study.
Three groups of patients were randomly assigned using computer-generated random numbers that were kept in sealed envelopes.• Group D: to receive intrathecal hyperbaric bupivacaine 0.5% (17.5 mg) 3.5 mL + 5 μg of dexmedetomidine in 0.5 mL saline.
- Group M: Patients received intrathecal hyperbaric bupivacaine 0.5% (17.5 mg) 3.5 mL + 2 mg of midazolam in 0.5 mL saline.
- Group C (control group): Patients received intrathecal hyperbaric bupivacaine 0.5% (17.5 mg) 3.5 mL + 0.5 mL saline.
The group-specific medication solutions were made by only the researchers and were handled in a closed envelope to another anesthesiologist who was not participating in the trial to inject it intrathecally. The patient's assigned group was concealed from the observing anesthesiologist, the patient, and the post-operative data collector. In this experiment, dexmedetomidine hydrochloride (Precedex®, Pfizer, Germany) was diluted in 20 mL saline 0.9% which makes each 0.5 mL to contain 5 µg dexmedetomidine. Midazolam hydrochloride (Midazolam-hameln, Suuny Pharmaceutical under license of Hameln Pharma GmbH Germany, Cairo, Egypt), and hyperbaric bupivacaine (Sunnypivacaine® Sunny Pharmaceuticals, Cairo, Egypt) were administered, midazolam presented in 5 mg ampoule in one mL diluted in 1.25 mL normal saline 0.9% to make 0.5 mL contain 2 mg midazolam.
2.1. Spinal anesthesia technique
Using a 25-gauge needle and 2 mL of 2% lignocaine, local infiltration was done to a sitting patient under aseptic conditions. Subsequently, with a midline approach and a 25-gauge Quincke spinal needle (Spinocan, B-Braun Melsungen AG, Melsungen, Germany), spinal anesthesia was delivered at the L4–L5 level. After appearance of CSF, 4 mL of the prepared solution was injected according to the selected group. Patients were then placed into supine positions. The sensory block was measured using a short bevel, 25G needle in the pin prick test, and the motor assessment was conducted using the Bromage scale.6
Lactated ringer solution was infused at a rate of 4 mL/kg/h. Hypotension was managed with 5 mg increments of ephedrine to keep mean arterial pressure (MAP) above 70 mmHg. If necessary, 0.5 mg of atropine was given to treat bradycardia, defined as a heart rate below 50 beats per min.
Duration of the sensory block, the primary outcome, was defined as the time period from completion of intrathecal drug administration, to 2-segment regression of sensory block. Secondary outcomes were set as: (i) Time to onset of sensory block (the period between the application of spinal anesthesia and the total loss of skin sensation to the pinprick test up to T10 level); (ii) Time to onset of motor block (described as the duration from the administration of spinal anesthesia to the extended modified Bromage scale of one, (iii) Motor block duration was specified as the duration from the delivery of spinal anesthesia to the extended modified Bromage scale of zero, (iv) perioperative HR and MAP, (v) shivering; the frequency and intensity of shivering using the Crossley and Mahajan scale,7 patient was regarded as shivering when scoring was 2 or more. (vi) Vomiting, (vii) Degree of sedation utilizing the Ramsay sedation scale.8 Patients were considered sedated when their score was 4 or greater.
2.2. Sample size calculation
A previous study by Aloka Samantaray,9 indicated the sensory block mean duration by dexmedetomidine was 286 min, for midazolam it was 236.9 min, and for the control group it was 212.7. To detect a real difference in means across groups with a power of 80% and a significance of 5% (two-sided), an overall sample size of 66 patients was required; i.e., 22 patients in all three groups. To allow for potential drop-outs; the overall sample size was 75 patients (25 patients in each group).
2.3. Statistical analysis
The data was tabulated using Microsoft Office Excel 2010 for Windows. Analysis was carried out using the IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp. The chi-square test was employed to analyze categorical variables, which were reported as frequencies (percentages).
The normality of the data distribution was established using the Shapiro-Wilk test. A repeated measures general linear model analysis of variance (ANOVA) was used to assess normally distributed data, which was subsequently provided as means and standard deviations (SD). Whenever data was determined not to be normally distributed, the Kruskal-Wallis test was employed, and the findings were displayed as medians and interquartile ranges (IQR). The Bonferroni test was carried out as a post-hoc test. A P < 0.05 was considered significant.
3. RESULTS
In the present trial 97 patients were examined for eligibility, 7 patients failed spinal anesthesia, and 15 patients refused to participate in the trial. Seventy-five adult patients were analyzed statistically (Figure 1).
The median age of enrolled patients was 44 years (IQR = 30.5–58.5). Regarding (ASA) physical condition classification, 57% of patients were ASA class I, 28% were class II, and just 14.7% were class III.
| Table 2: Comparative demographics of the three studied groups | ||||
| Parameters | Group D (n=25) | Group M (n=25) | Group C (n=25) | P value |
| Age (in years) | 46 (31–60) | 43 (28–55) | 45 (32–60) | 0.730 |
| Gender | ||||
| Male | 15 (60) | 17 (68) | 14 (56) | 0.675 |
| Female | 10 (40) | 8 (32) | 11 (44) | |
| ASA physical status | ||||
| I | 14 (56) | 12 (48) | 17 (68) | 0.256 |
| II | 8 (32) | 10 (40) | 3 (12) | |
| III | 3 (12) | 3 (12) | 5 (20) | |
| Type of operation | ||||
| Extended intra lesion curettage with cementing | 5 (20) | 5 (20) | 4 (16) | 0.743 |
| Tumor resection and vascularity grafting | 6 (24) | 5 (20) | 4 (16) | |
| Above knee amputation | 4 (16) | 7 (28) | 6 (24) | |
| Below knee amputation | 4 (16) | 4 (16) | 7 (28) | |
| Wide margin resection and recycling | 6 (24) | 4 (16) | 4 (16) | |
| Surgery duration (min) | 160 (130–220) | 170 (110–190) | 155 (100–180) | 0.166 |
| IV fluids (mL) | 2050 (1825–2900) | 2000 (1800–2300) | 1900 (1800–2400) | 0.428 |
| Values are expressed as Median (IQR), or N (%). P values for Chi–Square test and Kruskal–Wallis’s test. | ||||
| Table 3: Comparative sensory and motor blockade in the three studied groups | |||||
| Parameters | Group D
(n=25) |
Group M
(n=25) |
Group C
(n=25) |
P value | Post hoc |
| Time to reach sensory level T10 (min) | 3.5 (2–5) | 4 (4–5) | 4.5 (4–5) | 0.730 | |
| Time to reach motor block Bromage 1 level (min) | 8 (6–9) | 7 (6–8) | 10 (9–11) | 0.256 |
|
| Duration of motor blockade (min)* | 250 (209–324) | 153 (151–156) | 125 (102–141) | 0.026 | P1= 0.005 P2 < 0.001 P3 = 0.043 |
| Two–segment regression time (min)* | 132 (108–141) | 119 (109–126) | 98 (86–116) | < 0.001 | P1= 0.02 P2 =0.007 P3 = 0.029 |
| Values are shown as Median (IQR). P values for Kruskal–Wallis’s test. * Post-hoc tests showed that there were significant differences between all of the two-group comparisons (three comparisons) regarding duration of motor block and two-segment regression time. P1: P value between Group D and Group M. P2: P value between Group D and Group C. P3: P value between Group M and Group C. | |||||
The median duration of surgery was 163 min; the median time to achieve sensory level to T10 level was 4 min; and the median time to reach motor block level was 8 minas shown in Table 1. The comparison of the three study groups, was based on demographics showed no significant differences (P > 0.05) (Table 2).
The median duration of motor blockade was 250 min for Group D patients, 153 min for Group M patients, and 125 min for Group C patients, and this difference was statistically significant (P = 0.026). Post-hoc tests found significant differences among all of the comparisons between pairs.
| Table 4: Comparative intra-operative Ramsay’s sedation score at different time points | |||||
| Time | Score | Group D
(n=25) |
Group M (n=25) | Group C (n=25) | P value |
| Baseline | Score II | 25 (100) | 25 (100) | 25 (100) | ----- |
| 5 min | Score I | 25 (100) | 25 (100) | 25 (100) | ----- |
| 10 min
|
Score I Score III |
25 (100) 0 (0.00) |
24 (96) 1 (4) |
25 (100) 0 (0.00) |
0.363 |
| 15 min
|
Score I Score IV |
25 (100) 0 (0.00) |
24 (96) 1 (4) |
25 (100) 0 (0.00) |
0.363 |
| 20 min
|
Score I Score III Score IV |
24 (96) 1 (4) 0 (0.00) |
24 (96) 0 (0.00) 1 (4) |
25 (100) 0 (0.00) 0 (0.00) |
0.402 |
| 25 min
|
Score I Score III |
25 (100) 0 (0.00) |
24 (96) 1 (4) |
24 (96) 1 (4) |
0.598 |
| 30 min | Score I | 25 (100) | 25 (100) | 25 (100) | ----- |
| 45 min | Score I | 25 (100) | 25 (100) | 25 (100) | ----- |
| 60 min
|
Score I IV |
24 (96) 1 (4) |
25 (100) 0 (0.00) |
25 (100) 0 (0.00) |
0.363 |
| Values are showed as Number (%). P values for Chi-Square test. | |||||
The two-segment regression time was 132 min for Group D patients, 119 min in Group M patients, and 98 min in Group C patients, and this difference was statistically significant (P < 0.001). Post-hoc tests found significant differences among all of the pairwise comparisons. There were no significant differences in the median time to reach sensory level T10 or the median time to achieve motor block level among the studied groups (Table 3). There was a significant difference in the recorded heart rates among the groups in the time period between 15 min to 60 min following the start of the surgery. Post-hoc tests showed that heart rate was considerably higher in Group C, compared to Group D and M (Figure 2).
| Table 5: Comparison of intra-operative vomiting and shivering between the three studied groups. | ||||
| Complication | Group D
(n=25) |
Group M
(n=25) |
Group C
(n=25) |
P value |
| Vomiting | 3 (12) | 2 (8) | 1 (4) | 0.581 |
| Shivering
I II III |
0 (0.00) 3 (12) 0 (0.00) |
0 (0.00) 2 (8) 0 (0.00) |
0 (0.00) 2 (8) 3 (12) |
0.165 |
| Values are showed as Number (%). P values for Chi-Square test. | ||||
36% of patients in Group D experienced intraoperative bradycardia, 20% in Group M, and only 8% in Group C, and this difference was statistically significant (P = 0.0241) (Figure 3).
There was a significant difference in the recorded MAP among the three groups from 10 to 75 min and between 120 to 150 min after the start of the operation. MAP was significantly higher in Group C in comparison with the other two groups in the period 10 to 75 min and higher in Group M when compared to the other two groups in the period between 120 to 150 min (Figure 4).



Regarding the comparison of the recorded intraoperative hypotension at different time points, there was a significant difference in the recorded episodes of hypotension among the three groups in the time period between 15 min and 30 min and between 120 and 135 min after the start of the intrathecal injection. Post-hoc tests revealed that Groups D and M had considerably greater frequencies of hypotension episodes than Group C.
There were no significant differences among the three groups regarding SpO2 at any particular moment.
Ramsay’s sedation scores at different time points were not significantly difference among the three groups at any particular period (Table 4).
Vomiting was reported in 3 patients in Group D, 2 patients in Group M, and in one patient in Group C (Table 5). Shivering Grade II was documented in three patients in Group D, two patients in Group M, and two patients in Group C. Shivering in Grade III was noticed in three subjects in Group C. There were no significant differences between the three groups.
4. DISCUSSION
The most important finding of our study is both dexmedetomidine and midazolam prolonged both sensory and motor duration compared to control group with added privilege to dexmedetomidine. On the other hand, hypotension represents a minor complain in dexmedetomidine group compared to the other groups.
Spinal anesthesia is typically utilized for lower limb orthopedic surgery, including orthopedic cancer operations, since spinal anesthesia is a dependable and effective method with the advantage of postoperative analgesia and limited adverse effects. Adjuvants were used in conjunction with hyperbaric bupivacaine to extend the period of the sensory block, enhance postoperative analgesia, and increase the block's quality; however, these benefits came with a risk of hemodynamic instability, nausea, vomiting, and drowsiness.
Both midazolam and dexmedetomidine are fairly Recent entries to the list of adjuvants employed in spinal anesthesia and may function synergistically with intrathecal bupivacaine to lengthen the spinal anesthesia duration and for postoperative analgesia. When administered intrathecally, the two medications' mechanisms of action for producing antinociception are different.
Dexmedetomidine, an imidazoline molecule, is a D-isomer of dexmedetomidine, which is pharmacologically potent and displays specific α-2 adrenoceptor agonistic activity. Local anesthetics' motor and sensory block is extended by intrathecal α2-adrenoceptor agonists, which bind to postsynaptic dorsal horn neurons and presynaptic C-fibers. Depression of the release of C-fiber transmitters (substance P and glutamate) and postsynaptic dorsal horn neurons hyperpolarization cause this analgesic action.10
Al-Ghanem et al. performed a comparison between the effects of intrathecal 10 mg isobaric bupivacaine with 5μg dexmedetomidine and 25 mg fentanyl for vaginal hysterectomy. They found that the addition of 5 μg dexmedetomidine caused longer duration of motor and sensory block and fewer adverse reactions, such as bradycardia, hypotension, and pruritus, when compared to 25 μg fentanyl.11
In urological procedures, Al-Mustafa et al. evaluated the effects of 5 g and 10 g of dexmedetomidine with hyperbaric bupivacaine and showed that dexmedetomidine extends the time frame of spinal anesthesia in a dose-related manner.12
Kanazi GE et al. evaluated the impact of a low dose of dexmedetomidine (3 μg) compared to clonidine (30μg) when used in combination with bupivacaine intrathecally in transurethral resection of the prostate and bladder tumors. According to their findings, intrathecal dexmedetomidine had a quicker motor block onset and a longer duration of both motor and sensory block while maintaining hemodynamic stability and causing no sedation.13
The α-2 adrenergic agonist effects in the brain and spinal cord attenuate the sympathetic tone and decrease the catecholamines, thus avoiding vasoconstriction and increasing the threshold of shivering, so dexmedetomidine has anti-shivering features, as noticed by Usta B et al., who examined the impact of parenteral infusion of dexmedetomidine in the perioperative period compared to saline infusion during elective surgeries performed under spinal anesthesia and found that dexmedetomidine decreases perioperative incidence and severity of shivering.14
Hala et al. compared 10 μg and 15 μg of dexmedetomidine added to hyperbaric bupivacaine, and they determined that dexmedetomidine prolonged sensory and motor blockade and decreased postoperative pain and analgesic consumption when compared with the control group, with no side-effects between the three groups except for sedation with 15 μg.15
Local anesthetic drugs function by inhibiting sodium channels. The synergy between α2-adrenoceptor agonists and local anesthetics may prolong their action, whereas the attachment of α2-adrenoceptor agonists to motor neurons in the dorsal horn could extend the motor block of spinal anesthetics.5 Yegin et al. found that following perianal surgery, 2 mg of intrathecal midazolam produced mild drowsiness and had a longer postoperative analgesic effect than the control group. Midazolam, when given intrathecally, may cause exposure to analgesic systems influenced by GABA.16
Kohno et al. stated that the analgesic property of intrathecal midazolam is produced by an influence on the GABAergic spread in substantia gelatinosa neurons in spinal cord slices of adult rats.17 Intrathecal Midazolam plays a role in the outflow of endogenous opioids. Acting on spinal delta receptors, the antinociceptive impacts of morphine-like compounds are enhanced when intrathecal Midazolam is administered.17
Midazolam inhibits excitatory synaptic transmission by acting on the gamma aminobutyric acid type A/benzodiazepine receptor in interneurons, resulting in a decrease in the excitability of spinal dorsal horn neurons. Precautions must be made when administering intrathecal midazolam in clinical settings in humans because several studies have yielded inconsistent findings on the drug's possible neurotoxicity in animals. Few animal experiments have discovered histological proof of neurotoxicity in rats and rabbits following the administration of intrathecal midazolam.18, 19, 20 Other histological experiments in animals demonstrated that intrathecal midazolam does not generate notable alterations in the spinal cord.21, 22
Borg and Krijnen additionally stated that the continuous intrathecal delivery of midazolam to patients with resistant chronic benign pain produced quick and almost complete relief of pain with practically no adverse effects, and patients exhibited no tolerance to the midazolam analgesic effect.23
Shrivastav, R. et al. compared 5 μg dexmedetomidine with 1 mg of midazolam and the control group in any surgery done with spinal anesthesia. He observed that the beginning of the sensory block of dexmedetomidine is statistically faster than midazolam and control groups, while the midazolam group is statistically faster than the control group. The duration of sensory blockade by pin brick, dexmedetomidine, is statistically longer than midazolam and to control groups. The three groups had similar hemodynamics, but the dexmedetomidine group had more bradycardia.24
Aloka Samantaray et al. evaluated adding dexmedetomidine 5 μg vs. midazolam 1 mg to intrathecal bupivacaine in endourological procedures. The sensory block duration was observed to be statistically longer in the dexmedetomidine group than midazolam and the control group. Dexmedetomidine induced sedation in the first 30 min, with no nausea or vomiting. Dexmedetomidine generates hypotension in 35% of patients and bradycardia in 25% of patients, although the difference in hypotension or bradycardia was not significant.9
Al-Arnous MO, et al. compared intrathecal dexmedetomidine 5 μg and midazolam 2 mg with a control group in lower limb orthopedic procedures. While neither dexmedetomidine nor midazolam reduced blood pressure, they did lengthen sensory or motor duration; however, the dexmedetomidine group suffered more bradycardia than the midazolam group.25
In our current study, we examined the addition of 5 μg Dexmedetomidine against 2 mg midazolam to 0.5% hyperbaric bupivacaine in spinal anesthesia for lower limb orthopedic cancer procedures. The current study's primary outcome was the duration of sensory black.
Regarding the duration of sensory block, we used two segments regression time: the median duration of sensory block for the dexmedetomidine group is 132 min, which is statistically longer than midazolam and control groups; the median duration of sensory block for the midazolam group is 119 min, which is statistically longer than the control group, in which the median duration of sensory block is 98 min. The results we obtained are in line with those of Al-Arnous MO et al.25 and Shrivastav, R. et al.,24 but not with those of Aloka Samantaray et al.,9 who used only 1 mg of midazolam in contrast to our study, which used 2 mg. This difference may have contributed to our research's longer duration of sensory blockage.
The motor block for Group Dexmedetomidine has a median duration of 250 min. Which is significantly longer than midazolam and control groups; the median duration of motor block for the midazolam group is 153 min. Which is significantly greater than the control group, in which the median duration for motor block is 125 min.
There was not been a statistically significant variance in sensory or motor block onset among the three groups. Regarding the secondary outcome, there was no statistically significant variation in shivering or vomiting between the three groups. Most studies showed neither shivering nor vomiting.
For bradycardia, 36% of patients in the dexmedetomidine group had at least one episode of bradycardia, while only 20% of patients in the midazolam group had bradycardia and 8% of patients in the control group had bradycardia, which was statistically significant across the three groups.
In terms of hypotension, the dexmedetomidine group and the midazolam group were statistically significantly different from the control group. Unlike Aloka Samantaray et al.,9 Shrivastav, R. et al.,24 and Mahmoud Omar Al-Arnous.25 The sedation was monitored using the Ramsay sedation score. 4 patients scored III in Ramsay sedation scores in the midazolam group, whereas only 2 patients in the dexmedetomidine group were sedated and one patient in the control group; however, this difference did not appear significant statistically.
Relative to shivering and vomiting, there were no statistically significant variations across the three groups under assessment.
5. LIMITATIONS
Unfortunately, we were not able to perform serum level of both drugs to assess the degree of systemic absorption, which represent limitation to this study. We recommend this in future researches as it will make a solid conclusion about the exact mechanism of action of both drugs as adjuvant to bupivacaine in spinal anesthesia for prolongation of both sensory and motor duration.
6. CONCLUSION
When used as additives in spinal anesthesia, dexmedetomidine has longer sensory and motor block than midazolam, and both dexmedetomidine and midazolam were longer in duration than the control group, so we recommend that dexmedetomidine is more efficient for lengthy operations like orthopedic malignant surgeries and can be utilized for prolongation of postoperative analgesia despite the side effects like hypotension and bradycardia, which can be managed by fluids, vasopressors, and anticholinergic drugs.
7. Funding
No grants from public, commercial, or not-for-profit funding organizations were given to the writers.
8. Availability of data
Data can be obtained from the authors upon reasonable inquiry after approval of the National Cancer Institute, Cairo University, Egypt.
9. Conflict of interest
The authors declare that there is no conflict of interest with any financial entity regarding the material presented in the work.
10. Consent for publication
The writers take responsibility for sharing this content. This transfer of publication rights includes a non-exclusive license to reproduce and distribute the article, including copies, translations, photographic reproductions, microform, digital format (offline, online), or any other similar kind of replication.
11. Ethics approval
On March 14, 2021, the Institutional Review Board (IRB number AP2103-301-02) approved the study, which was then carried out at the National Cancer Institute Hospital.
12.Clinical trial registration
The study was registered with Clinicaltrials.gov with ID No. NCT06315634.
13. Authors contribution
MZ, MK: Conception of the idea
MK, EH, ME, DT and MZ: Design of the study, analysis of the data, writing the manuscript. data collection; revised, and approved the final manuscript.
14. REFERENCES
- Gupta A, Kamat H, Kharod U. Efficacy of intrathecal midazolam in potentiating the analgesic effect of intrathecal fentanyl in patients undergoing lower limb surgery. Anesth Essays Res. 2015;9(3):379–83. [PubMed] DOI: 4103/0259-1162.164650
- Nagiub G, Adel-Aziz M, Abdelrahim M. The effects of adding dexamethasone to epidural bupivacaine for lower limb orthopedic surgery. J Curr Med Res Pract. 2019;4(2):192. DOI: 4103/JCMRP.JCMRP_79_17
- Omar H, Aboella WA, Hassan MM, Hassan A, Hassan P, Elshall A, et al. Comparative study between intrathecal dexmedetomidine and intrathecal magnesium sulfate for the prevention of post-spinal anaesthesia shivering in uroscopic surgery: (RCT). BMC Anesthesiol. 2019;19(1):190. [PubMed] DOI: 1186/s12871-019-0853-0
- Wang J, Wang Z, Song X, Wang N. Dexmedetomidine versus magnesium sulfate as an adjuvant to local anesthetics in spinal anesthesia: a meta-analysis of randomized controlled trials. J Int Med Res. 2020;48(8):300060520946171. [PubMed] DOI: 1177/0300060520946171
- Sanwatsarkar S, Kapur S, Saxena D, Yadav G, Khan NN. Comparative study of caudal clonidine and midazolam added to bupivacaine during infra-umbilical surgeries in children. J Anaesthesiol Clin Pharmacol. 2017;33(2):241–7. [PubMed] DOI: 4103/0970-9185.209739
- Bhati K, Kuraning K, Dhawan S, Jawaid I, Khilji Y. Comparison of block characteristics of intrathecal isobaric levobupivacaine versus isobaric ropivacaine for infra-umbilical surgeries in school-age children: A prospective randomized double-blind study. Indian J Anaesth. 2021;65(9):662. [PubMed] DOI: 4103/ija.IJA_242_21
- Ahmed A, Aslam M. Prevention of shivering during lower segment cesarean section. Prof Med J. 2013;20(03):409–15. [FreeFullText]
- Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2:656–9. [PubMed] DOI: 1136/bmj.2.5920.656
- Samantaray A, Hemanth N, Gunnampati K, Pasupuleti H, Mukkara M, Rao MH. Comparison of the effects of adding dexmedetomidine versus midazolam to intrathecal bupivacaine on postoperative analgesia. Pain Physician. 2015;18(1):71–7. [PubMed]
- Eisenach JC, De Kock M, Klimscha W. α2-Adrenergic agonists for regional anesthesia: A clinical review of clonidine (1984–1995). Anesthesiology. 1996;85:655–74. [PubMed] DOI: 1097/00000542-199609000-00026
- Al-Ghanem SM, Massad IM, Al-Mustafa MM, Al-Zaben KR, Qudaisat IY, Qatawneh AM, et al. Effect of adding dexmedetomidine versus fentanyl to intrathecal bupivacaine on spinal block characteristics in gynecological procedures. Am J Appl Sci. 2009;6:882–7. DOI: 3844/ajassp.2009.882.887
- Al-Mustafa MM, Abu-Halaweh SA, Aloweidi AS, Murshidi MM, Ammari BA, Awwad ZM, et al. Effect of dexmedetomidine added to spinal bupivacaine for urological procedures. Saudi Med J. 2009;30:365–70. [PubMed]
- Kanazi GE, Aouad MT, Jabbour-Khoury SI, Al Jazzar MD, Alameddine MM, Al-Yaman R, et al. Effect of low-dose dexmedetomidine or clonidine on the characteristics of bupivacaine spinal block. Acta Anaesthesiol Scand. 2006;50(2):222–7. [PubMed] DOI: 1111/j.1399-6576.2006.00919.x
- Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A. Dexmedetomidine for the prevention of shivering during spinal anesthesia. Clinics (Sao Paulo). 2011;66(7):1187–91. [PubMed] DOI: 1590/s1807-59322011000700011
- Eid H, Shafie MA, Youssef H. Dose-related prolongation of hyperbaric bupivacaine spinal anesthesia by dexmedetomidine. Ain Shams J Anesthesiol. 2011;4(2):83–95.
- Yegin A, Sanli S, Dosemeci L, Kayacan N, Akbas M, Karsli B. The analgesic and sedative effects of intrathecal midazolam in perianal surgery. Eur J Anaesthesiol. 2004;21:658–62. [PubMed] DOI: 1017/s0265021504008129
- Kohno T, Wakai A, Ataka T, Ikoma M, Yamakura T, Baba H. Actions of midazolam on excitatory transmission in dorsal horn neurons of adult rat spinal cord. Anesthesiology. 2006;104:338–43. [PubMed] DOI: 1097/00000542-200602000-00020
- Malinovsky JM, Cozian A, Lepage JY, Mussini JM, Pinaud M, Souron R. Ketamine and midazolam neurotoxicity in the rabbit. Anesthesiology. 1991;75:991–7. [PubMed] DOI: 1097/00000542-199107000-00015
- Svensson BA, Welin M, Gordh T Jr, Westman J. Chronic subarachnoid midazolam (Dormicum) in the rat. Morphologic evidence of spinal cord neurotoxicity. Reg Anesth. 1995;20:426–34. [PubMed]
- Erdine S, Yucel A, Ozyalcin S, Ozyuvaci E, Talu GK, Ahiskali B, et al. Neurotoxicity of midazolam in the rabbit. Pain. 1999;80:419–23. [PubMed] DOI: 1016/s0304-3959(98)00240-1
- Schoeffler P, Auroy P, Bazin JE, Taxi J, Woda A. Subarachnoid midazolam: histologic study in rats and report of its effect on chronic pain in humans. Reg Anesth. 1991;16:329–32. [PubMed]
- Nishiyama T, Matsukawa T, Hanaoka K. Acute phase histopathological study of spinally administered midazolam in cats. Anesth Analg. 1999;89:717–20. [PubMed] DOI: 1097/00000539-199909000-00035
- Borg PA, Krijnen HJ. Long-term intrathecal administration of midazolam and clonidine. Clin J Pain. 1996;12(1):63–8. [PubMed] DOI: 1097/00002508-199603000-00012
- Shrivastav R, Kumbhare S, Khandelwal A, Vanjare H. Comparison of the effect of adding dexmedetomidine versus midazolam to intrathecal bupivacaine on the post-operative analgesia. Eur J Mol Clin Med. 2022;9(1):1225.
- Al-Arnous M, Aly AE, Abdo A, El Said A comparison between the effect of adding dexmedetomidine and midazolam to intrathecal bupivacaine on the quality of spinal block for orthopedic surgery. Zagazig Univ Med J. 2014;20(3):1–9. [FreeFullText]