Yunita Widyastuti1, Akhmad Yun Jufan2, Untung Widodo,3 Calcarina Fitriani Retno Wisudarti,4 Sudadi,5 Rizki Ahmad Fauzi6, Firman Ardiansyah7
Author affiliations:
Background & objective: Intensive care has been associated with high cost and resource-intensive medical care. Therefore, a risk prediction model is required to plan time allocation, human resources, and the required equipment. Various risk predictions for ICU mortality and ‘Prolonged Length of Stay’ (PLOS) scores are already available. Still, the established model, such as the APACHE IV score or SAPS II, sometimes became impractical since they required many laboratory parameters. A model based on co-morbidities and demographic factors may be more useful in limited resources setting. Hence, we developed a simple ICU mortality and PLOS risk prediction model based on co-morbidities and demographic data.
Methodology: This retrospective cohort study was performed to develop a risk scoring for mortality and PLOS, using data from Dr. Sardjito Hospital Yogyakarta database between January 01-December 31, 2019. Logistic regression and bootstrap methods were used to create a risk score for estimating the risk. The discrimination performance of the model was evaluated using the area under the curve (AUC) of the receiver operating characteristic (ROC). The Hosmer-Lemeshow test was employed to assess the model’s calibration.
Results: A total of 415 patients were included in this study. The risk factors for mortality were perioperative support medication, kidney failure, neurologic disorder, respiratory failure, and intraoperative blood transfusion. The mortality score of 6 was associated with a 100% probability of mortality. Medical cases, GCS < 8, vasoactive/inotropic medication, sepsis, respiratory failure, and kidney failure were the risk factors for PLOS. PLOS score of 3 was associated with a 100% probability of PLOS. The discrimination for either mortality or PLOS was considered excellent with the AUC (± 95% CI) for mortality 0.896 (0.853-0.94), while for PLOS 0.878 (0.80-0.90). The calibration test found that both models had good calibration with P values of 0.53 and 0.55 for mortality and PLOS, respectively.
Conclusion: The ‘Mortality and Prolonged Length of Stay Prediction Score’ based on co-morbidities and demographic data upon admission to ICU had good accuracy and can be applied as a potential new scoring system in healthcare institutions.
Abbreviations: APACHE- Acute Physiologic Assessment and Chronic Health Evaluation; AUC; Area Under the Curve GCS- Glasgow Coma Scale; ICU- Intensive Care Unit; PLOS- ‘Prolonged Length of Stay’; PRC- Packed Red Cells; SAPS- Simplified Acute Physiology Score
Keywords: Risk Scoring; Mortality; Prolonged Length of Stay; ICU
Citation: Widyastuti Y, Jufan AY, Widodo U, Wisudarti CFR, Sudadi, Fauzi RA, Ardiansyah F. A tertiary care center-based study of a novel ‘ICU Mortality and Prolonged Stay Risk Scoring System’. Anaesth. pain intensive care 2024;28(1):100−107; DOI: 10.35975/apic.v28i1.2382
Received: October 02, 2023; Reviewed: November 27, 2023; Accepted: December 17, 2023
The Intensive Care Unit (ICU) is a high-cost and resource-intensive treatment unit. One of the efforts to improve ICU service quality is by designing a risk prediction system for mortality and prolonged length of stay (PLOS). The scoring system is needed for comparative audit and service evaluation, more focused planning, assistance for the decision-maker, allocation plans for time, human resources, and equipments in ICU.1
Several instruments have been adopted to predict the outcomes of ICU patients as well as their survival rates while in the hospital. The most commonly utilized risk scoring systems in clinical practice to measure the disease's fatality rate is mortality risk estimations based on acute physiology scores such as ‘Simplified Acute Physiology Score’ (SAPS) and ‘Acute Physiology and Chronic Health Evaluation’ (APACHE) score. Both techniques are based on a logistic regression test of physiology-specific signals collected within the first day after ICU admission.2 In European and Asian countries, the APACHE and SAPS have been used to predict mortality and PLOS.3,4 However, they require extensive laboratory and vital sign data. The performance of APACHE IV and SAPS II varied in predicting mortality, and only had moderate accuracy in predicting PLOS.2,4,5
A locally developed risk prediction system for ICU mortality and PLOS based on co-morbidity and demographic data may be more applicable. Therefore, we aomed to design a risk prediction system based on simple variables to predict ICU mortality and length of stay in our hospital.
A single-center retrospective study was performed between January 1-December 31, 2019. Ethical clearance approval for the study was obtained from the Medical and Health Research Ethical Committee of the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada. The inclusion criteria were adult (≥18 y old) patients admitted to the ICU of Dr. Sardjito General Hospital. The exclusion criteria were: post-cardiac surgery, length of stay less than 24 hours, referred out of the hospital, and patients whose medical record data could not be collected during the sampling period. The dependent variable was mortality, defined as ICU mortality, and PLOS, defined as the length of ICU stay of more than seven days. The patients data collected included: demographic variables (age, gender, height, body mass index (BMI), comorbidity variables (cardiovascular disease, neurological disorder, heart failure, respiratory failure, kidney failure, sepsis, malignancy, Glasgow Coma Scale upon admission), history of readmission to ICU, and surgical variables (surgery urgency), treatments upon admission like packed red cell (PRC) transfusion and inotropic/vasopressor support, as well as mechanical ventilation were included.
The entire data set was used for model development since this strategy resulted in better predictive accuracy than data-splitting.6 Candidate variables were selected based on literature, clinical experience, and hypotheses regarding their relationship to the outcomes.
Demographic data were presented as mean and standard deviation for numeric variables and as a percentage for nominal or categorical data. Bivariate analyses were performed using the Student’s t-test for numeric data and the chi-square test for categorical/dichotomous data. Variables with P < 0.25 were candidates for the logistic regression test. Variables with P < 0.05 from univariable logistic regression were included in the multivariable analysis. Significant variables in the multivariate test and a constant formed a logistic regression equation formula. The bootstrapping method was employed for internal validation of the model. The risk score was developed based on the final logistic regression model using the method described by Sullivan et al.7 The lowest beta coefficient was used as the base point. The score was obtained by dividing the beta coefficients of each significant variable by the lowest beta coefficients. The score was then summed up and yielded a score for assessing the likelihood of mortality and PLOS.
The discriminatory performance of the model was evaluated using receiver operating characteristics (ROC) curves. The area under the curve (AUC or C-statistic) compared the discrimination between the various models. Values ≥ 0.7 were considered acceptable, and values ≥ 0.8 were good. The calibration was evaluated with the Hosmer–Lemeshow test by allocating patients to the predicted probability outcome. P > 0.05 indicated adequate goodness of fit. Statistical analysis was performed using the IBM SPSS software package (version 27 SPSS Inc., Chicago, IL).
A total of 420 patients were admitted to the ICU of a tertiary hospital between 1st January 2019 through 31st December 2019. Five patients were excluded for being less than 18 y old. The subject’s characteristics are presented in Table 1.
Of these 415 patients, 228 (54.9%) were women with a mean age of 41 ± 15 y. There were 109 deaths (26.3%), and PLOS was reported in 175 (41.7%) patients. Of 275 (66.2%) postsurgical cases, 25.8% were emergency surgery. A total of 342 patients (82.4%) received mechanical ventilation support during the ICU stay.
Table 1 presents the variables related to mortality and PLOS. Variables with P < 0.25, were emergency surgery, perioperative support medication, sepsis, cardiovascular disease, neurological disorder, heart failure, kidney failure, respiratory failure, perioperative transfusion, gender, age, and BMI.
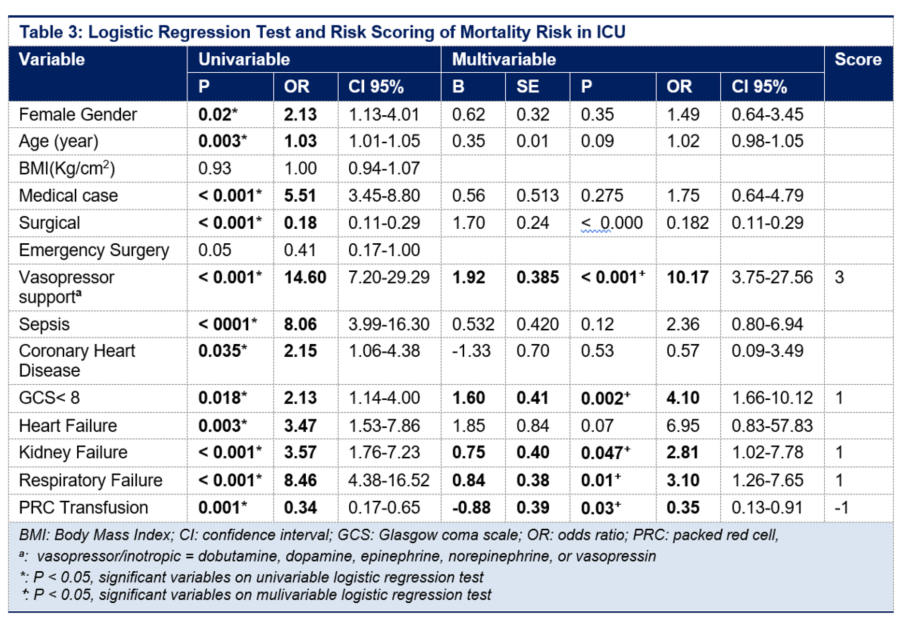
Table 2, in the bivariate test, was included in logistic regression test. The logistic regression for mortality model is presented in Table 2. From the univariable logistic regression test and followed by the multivariable logistic regression test, we found that vasopressor/inotropic support, low GCS, respiratory failure, kidney failure, and intraoperative PRC transfusion therapy were identified as predictive factors for ICU mortality.
The cut-off points show that a mortality score of 4 was associated with a mortality probability of 84%, while a score of 6 was associated with a mortality probability of 100%. A mortality score of -1 was associated with a 2.7% mortality probability, and each one-point increase corresponds to a 6.4%, 25%, 36%, 47.8%, 84%, 85.7%, and 100% mortality probability.
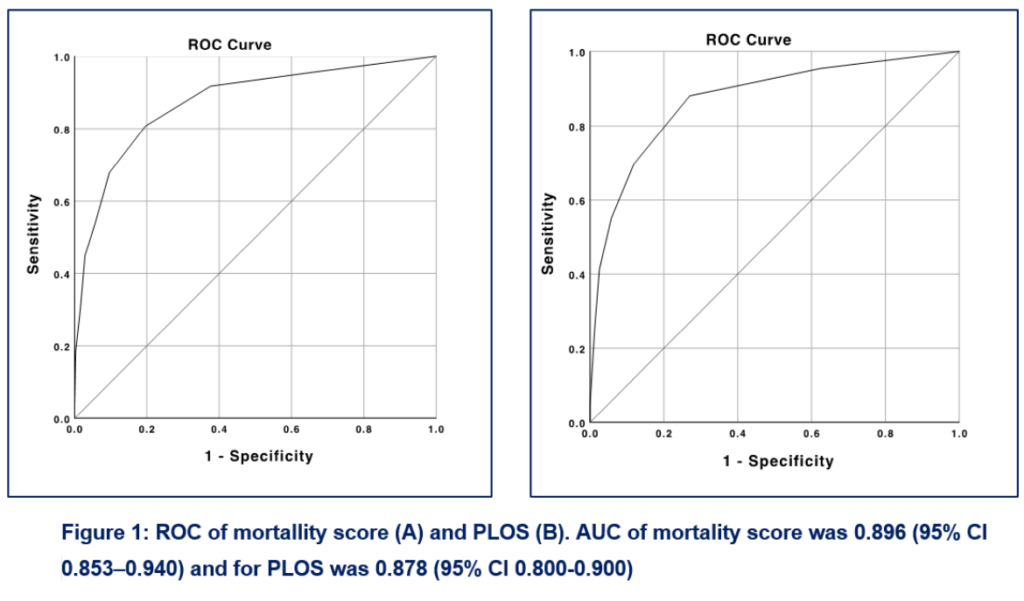
The mortality prediction model yielded an AUC of 0.896 (95% CI 0.853-0.940), indicating excellent discrimination performance, with an accuracy of 89.6%, as shown in Figure 1A. The Hosmer-Lemeshow test found that the mortality score had good calibration in predicting mortality, indicating no difference between predicted and actual mortality (P = 0.53).
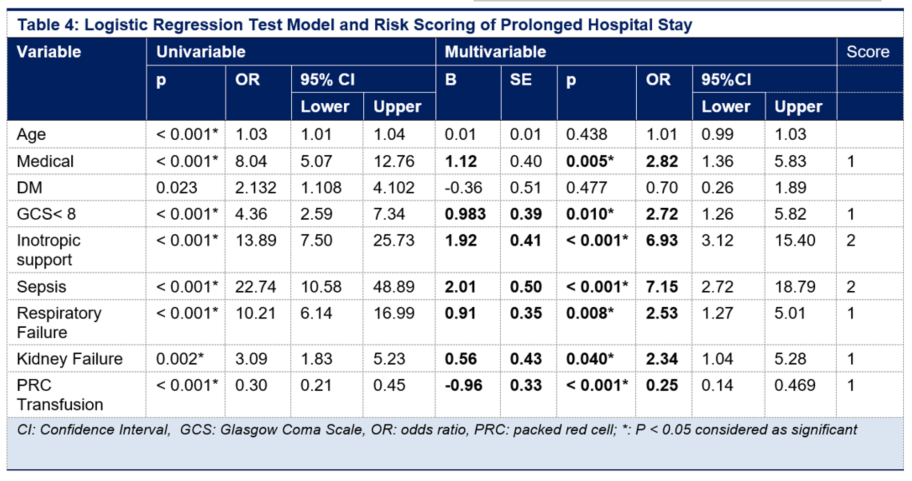
For PLOS, multivariable logistic regression test found that medical case, GCS < 8, vasoactive/inotropic drugs, sepsis, respiratory failure, kidney failure were the predictors of PLOS in ICU; while transfusion was a protective factor of PLOS with OR < 1 (95% CI). Based on significant variables from multivariable analysis, therefore mortality and PLOS prediction scores were made (Table 3 and 4).
A PLOS score of 3 was associated with 100% PLOS probability. PLOS score of -1 was associated with a 12.8% probability of PLOS, and each increase of one point correlated to 17.2%, 33.9%, 63.3%, and 100% probability of PLOS, respectively.
The PLOS score model also had an excellent discriminatory performance. PLOS score had an AUC of 0.878 (CI 95%; 0.800-0.900) (Figure 1B). Results from the Hosmer-Lemeshow test suggested that the PLOS score had good calibration (P = 0.50).
4.1. Mortality Prediction Model
Predicting mortality in ICU patients is critical for assessing illness severity and the risk and benefit of potential treatments, interventions, and healthcare policies. Most ICU mortality prediction scores, such as the APACHE IV and the SAPS II, demonstrated good accuracy.2,8 However, their complexity can be cumbersome in ICUs with limited resources in developing countries.
This study discovered that a mortality prediction score developed from the ICU database could perform accurately with excellent discrimination (AUC 0.896; 95% CI 0.853-0.940) and calibration performance.
Intraoperative vasopressor/inotrope use, low GCS, respiratory failure, kidney failure, and intraoperative red cell transfusion therapy were the independent variables. A cut-off point score of more than three is associated with a high mortality rate.
The variables in our mortality prediction score have also been identified as independent predictors of ICU mortality in other studies. Vasopressor/inotropic support has been associated with a high probability of ICU mortality.9,10 Multiple organ dysfunction score (MODS), Sequential organ failure assessment (SOFA), and SAPS II scores also included low GCS, acute respiratory failure, and kidney failure.11–13
Patients with norepinephrine ≥0.7 μg/kg/min had a relative risk of 9.353 and were independent predictors of ICU death.14 Inotropic or vasopressor support has been associated with increased myocardial oxygen consumption, myocardial ischemia, and arrhythmias in critically ill patients.15
Vasopressors and inotropes treatment for critically ill patients has been associated with the potential risk of decreased renal and visceral blood flow. It is based on the rationale that noradrenaline increases MAP through vasoconstriction via α-adrenergic stimulation, and excessive vasoconstriction in regional vascular beds may decrease organ blood flow, especially in the kidney. However, the data is somewhat lacking, and the evidence may suggest otherwise. Restoring blood pressure with norepinephrine may even improve microvascular flow and tissue oxygenation in pathological vasodilation.16
In acute myocard infarct complicated by shock, inotropic agents preserve noninfarcted myocardial cells by improving their mitochondrial function. However, dopamine may increase the already high cytosolic Ca2+ in cardiac myocytes post-ischemia. This, in turn, activates proteolytic enzymes, proapoptotic signal cascades, mitochondrial damage, and cell necrosis. Therefore, clinicians should administer the lowest possible doses of inotropic and vasopressors that optimize vital tissue perfusion while preventing potential adverse events.15
Kidney failure has been associated with many complications. Erythropoietin, a hormone secreted mainly by the kidneys, stimulates red blood cell production. Kidney failure may result in reduced production and anemia. Chronic Kidney Disease (CKD) may also harm the cardiovascular system. Hypertension and fluid excess are two other factors that can aggravate heart function and lead to congestive heart failure. CKD is also linked to uremic syndrome, electrolyte disorders such as potassium retention, and metabolic acidosis, all of which can be fatal.17,18
Neurological disorders defined as impaired consciousness based on GCS were also identified as an independent mortality predictor. Nik et al.19 found that low GCS was associated with a higher risk of mortality and the discrimination ability was comparable with APACHE II score. Low GCS has been associated with the risk of aspiration pneumonia, which may lead to respiratory failure. Therefore, it is essential to identify low GCS to prevent the development of aspiration pneumonia.
Perioperative blood transfusion was identified as a protective variable against mortality in this study. Blood transfusion was reported to have an association with lower patient mortality in the ICU in a study of 4,470 critically ill patients. When compared to anemic patients who were not transfused, patients who received 1 to 3 blood units had an adjusted odds ratio (OR) of 0.61 (95% CI; 0.37-1.00, P = 0.026) and 0.49 (95% Cl; 0.23-1.03, P = 0.03).20 PRC transfusion is commonly administered in critically ill patients to increase oxygen delivery and oxygenation, especially in shock patients. The use of transfusion is justified because an increase in hemoglobin improves blood oxygen transport capacity, allowing for more oxygen supply to oxygen-dependent tissues.21
4.2. PLOS Prediction Model
This study found a highly accurate PLOS prediction score. A cut-off score of > 2 was associated with a high probability of mortality (> 84%). The score had excellent discrimination performance and calibration. Intraoperative vasopressor/inotropic usage, low GCS, respiratory failure, kidney failure, and intraoperative PRC transfusion were identified as predictive mortality factors in ICU. The use of vasopressor/inotropic consistenly had high score in predicting both mortality and PLOS in our population.
Several studies found various predictors of ICU PLOS including age, comorbidity, prolonged mechanical ventilation, sepsis and laboratory finding. Renal failure, the use of vasopressor/inotropic, and mechanical ventilator are the most predictor found in previous studies.22–25
APACHE IV seems to have less accuracy to predict PLOS in ICU despite the good discrimination and calibration in predicting mortality in our population. The AUC for predicting PLOS is 0.68 (0.62–0.74) and p value =0.01 for Hosmer-Lemeshow test for calibration, which considered poor.5
Different study design, sample size, population characteristics, operational definitions, inclusion and exclusion criteria and different methods of modelling may affect the results. Some studies used different definitions for prolonged ICU length of stay varied, for instance 14 days in Tobi and Amadasun, 30 days in Cevic et al. For the current study, we defined ICU prolonged length of stay as >7 ICU days, similar used in a study by Bohmer et al.25 Despite different characteristic of the population, methods, and operational definition of PLOS we found common variables predicting PLOS in ICU.
Perioperative vasoactive/inotropic agents support, neurological disorder, respiratory failure, kidney failure, and perioperative blood transfusion were predictors of ICU mortality. Meanwhile, medical cases, GCS <8, vasoactive/inotropic support, sepsis, respiratory failure, kidney failure, and transfusion were predictors of ICU PLOS. Both ICU mortality and PLOS score had excellent discrimination performance.
6. Data availability
Numerical data generated in the course of this study is available with the correspondence author.
7. Acknowledgements
A part of this study utilized data from the theses of Rizky Ahmad Fauzy and Firman Ardiansyah.
8. Conflicts of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
9. Authors’ contribution
All authors contributed in the preparation of the study protocol, conduct of the study, literature search and manuscript preparation.
Author affiliations:
- Yunita Widyastuti, Department of Anesthesiology and Intensive Therapy, Dr. Sardjito General Hospital, Yogyakarta, Indonesia/Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; E-mail: yunita.widya@ugm.ac.id; ORCID: {0000-0002-9717-2030}
- Akhmad Yun Jufan, Department of Anesthesiology and Intensive Therapy, Dr. Sardjito General Hospital, Yogyakarta, Indonesia/Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; E-mail: jufan@ugm.ac.id
- Untung Widodo, Department of Anesthesiology and Intensive Therapy, Dr. Sardjito General Hospital, Yogyakarta, Indonesia/Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; E-mail: untung-wid@ugm.ac.id
- Calcarina Fitriani Retno Wisudarti, Department of Anesthesiology and Intensive Therapy, Dr. Sardjito General Hospital, Yogyakarta, Indonesia/Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; E-mail: calcarina.wisudarti@ugm.ac.id
- Sudadi, Department of Anesthesiology and Intensive Therapy, Dr. Sardjito General Hospital, Yogyakarta, Indonesia/Faculty of Medicine, Public Health and Nursing, Universitas Gadjah Mada, Yogyakarta, Indonesia; E-mail: dsudadi@ugm.ac.id
- Rizki Ahmad Fauzi, Anesthesiologist, Pupuk Kaltim Hospital Bontang, East Kalimantan, Indonesia; E-mail: rizqibontang29@gmail.com
- Firman Ardiansyah, Anesthesiologist, RSUD Sleman Yogyakarta, Indonesia; E-mail: firmananestesi@gmail.com
ABSTRACT
Background & objective: Intensive care has been associated with high cost and resource-intensive medical care. Therefore, a risk prediction model is required to plan time allocation, human resources, and the required equipment. Various risk predictions for ICU mortality and ‘Prolonged Length of Stay’ (PLOS) scores are already available. Still, the established model, such as the APACHE IV score or SAPS II, sometimes became impractical since they required many laboratory parameters. A model based on co-morbidities and demographic factors may be more useful in limited resources setting. Hence, we developed a simple ICU mortality and PLOS risk prediction model based on co-morbidities and demographic data.
Methodology: This retrospective cohort study was performed to develop a risk scoring for mortality and PLOS, using data from Dr. Sardjito Hospital Yogyakarta database between January 01-December 31, 2019. Logistic regression and bootstrap methods were used to create a risk score for estimating the risk. The discrimination performance of the model was evaluated using the area under the curve (AUC) of the receiver operating characteristic (ROC). The Hosmer-Lemeshow test was employed to assess the model’s calibration.
Results: A total of 415 patients were included in this study. The risk factors for mortality were perioperative support medication, kidney failure, neurologic disorder, respiratory failure, and intraoperative blood transfusion. The mortality score of 6 was associated with a 100% probability of mortality. Medical cases, GCS < 8, vasoactive/inotropic medication, sepsis, respiratory failure, and kidney failure were the risk factors for PLOS. PLOS score of 3 was associated with a 100% probability of PLOS. The discrimination for either mortality or PLOS was considered excellent with the AUC (± 95% CI) for mortality 0.896 (0.853-0.94), while for PLOS 0.878 (0.80-0.90). The calibration test found that both models had good calibration with P values of 0.53 and 0.55 for mortality and PLOS, respectively.
Conclusion: The ‘Mortality and Prolonged Length of Stay Prediction Score’ based on co-morbidities and demographic data upon admission to ICU had good accuracy and can be applied as a potential new scoring system in healthcare institutions.
Abbreviations: APACHE- Acute Physiologic Assessment and Chronic Health Evaluation; AUC; Area Under the Curve GCS- Glasgow Coma Scale; ICU- Intensive Care Unit; PLOS- ‘Prolonged Length of Stay’; PRC- Packed Red Cells; SAPS- Simplified Acute Physiology Score
Keywords: Risk Scoring; Mortality; Prolonged Length of Stay; ICU
Citation: Widyastuti Y, Jufan AY, Widodo U, Wisudarti CFR, Sudadi, Fauzi RA, Ardiansyah F. A tertiary care center-based study of a novel ‘ICU Mortality and Prolonged Stay Risk Scoring System’. Anaesth. pain intensive care 2024;28(1):100−107; DOI: 10.35975/apic.v28i1.2382
Received: October 02, 2023; Reviewed: November 27, 2023; Accepted: December 17, 2023
1. INTRODUCTION
The Intensive Care Unit (ICU) is a high-cost and resource-intensive treatment unit. One of the efforts to improve ICU service quality is by designing a risk prediction system for mortality and prolonged length of stay (PLOS). The scoring system is needed for comparative audit and service evaluation, more focused planning, assistance for the decision-maker, allocation plans for time, human resources, and equipments in ICU.1
Several instruments have been adopted to predict the outcomes of ICU patients as well as their survival rates while in the hospital. The most commonly utilized risk scoring systems in clinical practice to measure the disease's fatality rate is mortality risk estimations based on acute physiology scores such as ‘Simplified Acute Physiology Score’ (SAPS) and ‘Acute Physiology and Chronic Health Evaluation’ (APACHE) score. Both techniques are based on a logistic regression test of physiology-specific signals collected within the first day after ICU admission.2 In European and Asian countries, the APACHE and SAPS have been used to predict mortality and PLOS.3,4 However, they require extensive laboratory and vital sign data. The performance of APACHE IV and SAPS II varied in predicting mortality, and only had moderate accuracy in predicting PLOS.2,4,5
A locally developed risk prediction system for ICU mortality and PLOS based on co-morbidity and demographic data may be more applicable. Therefore, we aomed to design a risk prediction system based on simple variables to predict ICU mortality and length of stay in our hospital.
2. METHODOLOGY
A single-center retrospective study was performed between January 1-December 31, 2019. Ethical clearance approval for the study was obtained from the Medical and Health Research Ethical Committee of the Faculty of Medicine, Public Health, and Nursing, Universitas Gadjah Mada. The inclusion criteria were adult (≥18 y old) patients admitted to the ICU of Dr. Sardjito General Hospital. The exclusion criteria were: post-cardiac surgery, length of stay less than 24 hours, referred out of the hospital, and patients whose medical record data could not be collected during the sampling period. The dependent variable was mortality, defined as ICU mortality, and PLOS, defined as the length of ICU stay of more than seven days. The patients data collected included: demographic variables (age, gender, height, body mass index (BMI), comorbidity variables (cardiovascular disease, neurological disorder, heart failure, respiratory failure, kidney failure, sepsis, malignancy, Glasgow Coma Scale upon admission), history of readmission to ICU, and surgical variables (surgery urgency), treatments upon admission like packed red cell (PRC) transfusion and inotropic/vasopressor support, as well as mechanical ventilation were included.
The entire data set was used for model development since this strategy resulted in better predictive accuracy than data-splitting.6 Candidate variables were selected based on literature, clinical experience, and hypotheses regarding their relationship to the outcomes.
Demographic data were presented as mean and standard deviation for numeric variables and as a percentage for nominal or categorical data. Bivariate analyses were performed using the Student’s t-test for numeric data and the chi-square test for categorical/dichotomous data. Variables with P < 0.25 were candidates for the logistic regression test. Variables with P < 0.05 from univariable logistic regression were included in the multivariable analysis. Significant variables in the multivariate test and a constant formed a logistic regression equation formula. The bootstrapping method was employed for internal validation of the model. The risk score was developed based on the final logistic regression model using the method described by Sullivan et al.7 The lowest beta coefficient was used as the base point. The score was obtained by dividing the beta coefficients of each significant variable by the lowest beta coefficients. The score was then summed up and yielded a score for assessing the likelihood of mortality and PLOS.
The discriminatory performance of the model was evaluated using receiver operating characteristics (ROC) curves. The area under the curve (AUC or C-statistic) compared the discrimination between the various models. Values ≥ 0.7 were considered acceptable, and values ≥ 0.8 were good. The calibration was evaluated with the Hosmer–Lemeshow test by allocating patients to the predicted probability outcome. P > 0.05 indicated adequate goodness of fit. Statistical analysis was performed using the IBM SPSS software package (version 27 SPSS Inc., Chicago, IL).
3. RESULTS
A total of 420 patients were admitted to the ICU of a tertiary hospital between 1st January 2019 through 31st December 2019. Five patients were excluded for being less than 18 y old. The subject’s characteristics are presented in Table 1.
Of these 415 patients, 228 (54.9%) were women with a mean age of 41 ± 15 y. There were 109 deaths (26.3%), and PLOS was reported in 175 (41.7%) patients. Of 275 (66.2%) postsurgical cases, 25.8% were emergency surgery. A total of 342 patients (82.4%) received mechanical ventilation support during the ICU stay.
Table 1 presents the variables related to mortality and PLOS. Variables with P < 0.25, were emergency surgery, perioperative support medication, sepsis, cardiovascular disease, neurological disorder, heart failure, kidney failure, respiratory failure, perioperative transfusion, gender, age, and BMI.
| Table 1: Patients’ Characteristics in relation to mortality | |||||
| Variable | Total | Survived | Died | p | |
| Gender | F | 228 (54.94) | 183 (59.80) | 45 (41.28) | 0.027* |
| M | 187 (45.06) | 123 (40.20) | 64 (58.72) | ||
| Age (year) | 41.74 ± 15.69 | 39.11 ± 14.35 | 46.09 ± 16.65 | 0.003* | |
| Weight (kg) | 60.42 ± 13.40 | 60.46 ± 13.19 | 60.38 ± 15.79 | 0.970 | |
| Height (cm) | 160.91 ± 8.93 | 160.87 ± 8.01 | 159.12 ± 15.97 | 0.262 | |
| BMI (kg/m2) | 23.20 ± 4.57 | 23.27 ± 4.65 | 23.33 ± 4.62 | 0.005* | |
| Surgical case | 275 (66.2) | 237 (86.2) | 38(13.8) | < 0.001* | |
| Emergency surgery | 107 (25.8) | 83 (77.5) | 24(22.4) | 0.068* | |
| PRC transfusion | 216(52.5) | 182 (84.3) | 34(15.7) | 0.001* | |
| Vasopressor supporta | 94 (22.7) | 31 (33.0) | 63 (67.0) | < 0.001* | |
| Sepsis | 84 (20.3) | 28 (33.3) | 56 (66.7) | < 0.001* | |
| Coronary Heart Disease | 69(16.7) | 48 (74.2) | 21(25.8) | 0.053* | |
| Low GCS | 81 (19.5) | 34 (42.0) | 47 (58.0) | 0.00* | |
| Heart Failure | 37(8.9) | 23(62.2) | 14(13.1) | 0.114* | |
| Kidney Failure | 73 (17.6) | 37 (50.7) | 36 (49.3) | < 0.001* | |
| Respiratory Failure | 117 (28.3) | 52 (44.4) | 65(55.6) | < 0.001* | |
| Malignancy | 95(22.9) | 72 (87.7) | 23(12.3) | 0.790 | |
| Neurological disorder | 184 (44.4) | 122 (66.3) | 62(33.7) | 0.041* | |
| Diabetes Mellitus | 41 (9.8) | 23 (56.1) | 18(43.9) | 0.008* | |
| Mechanical ventilation | 342 (82.4) | 247 (72.2) | 95(27.8) | 0.055* | |
| Readmission | 12 (2.9) | 10 (83.3) | 2(16.7) | 0.74 | |
| Prolonged Length of Stay | 175 (42.2) | 66(38.2) | 107(61.8) | < 0.001 | |
| Mortality rate | 109 (26.3) | ||||
| Data presented as mean ± SD or n (%);* P < 0.25, included in univariable logistic regression test
BMI: Body Mass Index, GCS: Glasgow Coma Scale, PLOS: Prolonged Length of Stay a: vasopressor = dobutamine, dopamine, epinephrine, norepinephrine, or vasopressin |
|||||
Table 2, in the bivariate test, was included in logistic regression test. The logistic regression for mortality model is presented in Table 2. From the univariable logistic regression test and followed by the multivariable logistic regression test, we found that vasopressor/inotropic support, low GCS, respiratory failure, kidney failure, and intraoperative PRC transfusion therapy were identified as predictive factors for ICU mortality.
| Table 2: Patients’ Characteristics in relation to Prolonged Length of Stay (PLOS) | |||||
| Variable | Total | No PLOS | PLOS | P-value | |
| Gender | F | 228 (54.94) | 137(57.08) | 91(52.0) | 0.053 |
| M | 187 (45.06) | 103(42.92) | 84(48.0) | ||
| Age (y) | 41.74 ± 15.69 | 39.29 ± 14.14 | 45.16 ± 17.08 | < 0.001* | |
| Weight (Kg) | 60.42 ± 13.40 | 60.76 ± 13.34 | 59.94 ± 13.49 | 0.539 | |
| Height (cm) | 160.91 ± 8.93 | 160.88 ± 8.42 | 160.95 ± 9.61 | 0.935 | |
| BMI (Kg/m2) | 23.20 ± 4.57 | 23.38 ± 4.77 | 22.94 ± 4.27 | 0.326 | |
| Surgical case | 274 (66.2) | 204(74.5) | 70(25.5) | < 0.001* | |
| Emergency surgery | 102 (25.3) | 61(58.1) | 44(41.9) | 1.000 | |
| PRC transfusion | 216(52.0) | 155(71.8) | 61(28.2) | < 0.001* | |
| Vasopressor support a | 94 (22.6) | 14(14.9) | 80(85.1) | < 0.001* | |
| Sepsis | 84 (20.2) | 8(9.5) | 76(90.5) | < 0.001* | |
| Coronary Heart Disease | 69(16.7) | 35(50.7) | 34(49.3) | 0.258 | |
| Low GCS | 81 (19.6) | 23(28.4) | 58(71.6) | < 0.001* | |
| Heart Failure | 37(8.9) | 14 (37.8) | 23 (62.2) | 0.014* | |
| Kidney Failure | 73 (17.6) | 26(35.6) | 47(64.4) | < 0.001* | |
| Respiratory Failure | 117 (28.3) | 25(21.4) | 92(78.6) | < 0.001* | |
| Malignancy | 95(22.9) | 62(65.3) | 34(34.7) | 0.157* | |
| Neurological disorder | 184 (44.4) | 95(51.6) | 89(48.4) | 0.016 | |
| Diabetes Mellitus | 41 (9.9) | 17(41.5) | 24(58.5) | 0.029* | |
| Mechanical ventilation | 342 (82.6) | 195(57.0) | 147(43.0) | 0.294 | |
| Readmission | 12 (2.9) | 9(75.0) | 3(25.0) | 0.235 | |
| PLOS | 175 (41.7) | ||||
| Mortality rate | 109 (26.0) | 0 | 109(100) | < 0.001 | |
| Data presented as mean ± SD or n (%);* P < 0.25, included in univariable logistic regression test
BMI: Body Mass Index, GCS: Glasgow Coma Scale, PLOS: Prolonged Length of Stay a: vasopressor = dobutamine, dopamine, epinephrine, norepinephrine, or vasopressin |
|||||
The cut-off points show that a mortality score of 4 was associated with a mortality probability of 84%, while a score of 6 was associated with a mortality probability of 100%. A mortality score of -1 was associated with a 2.7% mortality probability, and each one-point increase corresponds to a 6.4%, 25%, 36%, 47.8%, 84%, 85.7%, and 100% mortality probability.
The mortality prediction model yielded an AUC of 0.896 (95% CI 0.853-0.940), indicating excellent discrimination performance, with an accuracy of 89.6%, as shown in Figure 1A. The Hosmer-Lemeshow test found that the mortality score had good calibration in predicting mortality, indicating no difference between predicted and actual mortality (P = 0.53).
For PLOS, multivariable logistic regression test found that medical case, GCS < 8, vasoactive/inotropic drugs, sepsis, respiratory failure, kidney failure were the predictors of PLOS in ICU; while transfusion was a protective factor of PLOS with OR < 1 (95% CI). Based on significant variables from multivariable analysis, therefore mortality and PLOS prediction scores were made (Table 3 and 4).
A PLOS score of 3 was associated with 100% PLOS probability. PLOS score of -1 was associated with a 12.8% probability of PLOS, and each increase of one point correlated to 17.2%, 33.9%, 63.3%, and 100% probability of PLOS, respectively.
The PLOS score model also had an excellent discriminatory performance. PLOS score had an AUC of 0.878 (CI 95%; 0.800-0.900) (Figure 1B). Results from the Hosmer-Lemeshow test suggested that the PLOS score had good calibration (P = 0.50).
4. DISCUSSION
4.1. Mortality Prediction Model

Predicting mortality in ICU patients is critical for assessing illness severity and the risk and benefit of potential treatments, interventions, and healthcare policies. Most ICU mortality prediction scores, such as the APACHE IV and the SAPS II, demonstrated good accuracy.2,8 However, their complexity can be cumbersome in ICUs with limited resources in developing countries.
This study discovered that a mortality prediction score developed from the ICU database could perform accurately with excellent discrimination (AUC 0.896; 95% CI 0.853-0.940) and calibration performance.

| Table 5: Mortality and PLOS Score | |
| Mortality Score | |
| Predictors | Scores |
| Vasopressor/ inotropic support | 3 |
| GCS < 8 | 1 |
| Respiratory Failure | 1 |
| Kidney failure | 1 |
| Transfusion of PRC | -1 |
| PLOS Score | |
| Medical case | 1 |
| GCS < 8 | 1 |
| Vasopressor/ inotropic support | 2 |
| Sepsis | 2 |
| Respiratory Failure | 1 |
| Kidney Failure | 1 |
| Transfusion of PRC | -1 |
Intraoperative vasopressor/inotrope use, low GCS, respiratory failure, kidney failure, and intraoperative red cell transfusion therapy were the independent variables. A cut-off point score of more than three is associated with a high mortality rate.
The variables in our mortality prediction score have also been identified as independent predictors of ICU mortality in other studies. Vasopressor/inotropic support has been associated with a high probability of ICU mortality.9,10 Multiple organ dysfunction score (MODS), Sequential organ failure assessment (SOFA), and SAPS II scores also included low GCS, acute respiratory failure, and kidney failure.11–13

Patients with norepinephrine ≥0.7 μg/kg/min had a relative risk of 9.353 and were independent predictors of ICU death.14 Inotropic or vasopressor support has been associated with increased myocardial oxygen consumption, myocardial ischemia, and arrhythmias in critically ill patients.15
Vasopressors and inotropes treatment for critically ill patients has been associated with the potential risk of decreased renal and visceral blood flow. It is based on the rationale that noradrenaline increases MAP through vasoconstriction via α-adrenergic stimulation, and excessive vasoconstriction in regional vascular beds may decrease organ blood flow, especially in the kidney. However, the data is somewhat lacking, and the evidence may suggest otherwise. Restoring blood pressure with norepinephrine may even improve microvascular flow and tissue oxygenation in pathological vasodilation.16
In acute myocard infarct complicated by shock, inotropic agents preserve noninfarcted myocardial cells by improving their mitochondrial function. However, dopamine may increase the already high cytosolic Ca2+ in cardiac myocytes post-ischemia. This, in turn, activates proteolytic enzymes, proapoptotic signal cascades, mitochondrial damage, and cell necrosis. Therefore, clinicians should administer the lowest possible doses of inotropic and vasopressors that optimize vital tissue perfusion while preventing potential adverse events.15
Kidney failure has been associated with many complications. Erythropoietin, a hormone secreted mainly by the kidneys, stimulates red blood cell production. Kidney failure may result in reduced production and anemia. Chronic Kidney Disease (CKD) may also harm the cardiovascular system. Hypertension and fluid excess are two other factors that can aggravate heart function and lead to congestive heart failure. CKD is also linked to uremic syndrome, electrolyte disorders such as potassium retention, and metabolic acidosis, all of which can be fatal.17,18
Neurological disorders defined as impaired consciousness based on GCS were also identified as an independent mortality predictor. Nik et al.19 found that low GCS was associated with a higher risk of mortality and the discrimination ability was comparable with APACHE II score. Low GCS has been associated with the risk of aspiration pneumonia, which may lead to respiratory failure. Therefore, it is essential to identify low GCS to prevent the development of aspiration pneumonia.
Perioperative blood transfusion was identified as a protective variable against mortality in this study. Blood transfusion was reported to have an association with lower patient mortality in the ICU in a study of 4,470 critically ill patients. When compared to anemic patients who were not transfused, patients who received 1 to 3 blood units had an adjusted odds ratio (OR) of 0.61 (95% CI; 0.37-1.00, P = 0.026) and 0.49 (95% Cl; 0.23-1.03, P = 0.03).20 PRC transfusion is commonly administered in critically ill patients to increase oxygen delivery and oxygenation, especially in shock patients. The use of transfusion is justified because an increase in hemoglobin improves blood oxygen transport capacity, allowing for more oxygen supply to oxygen-dependent tissues.21
4.2. PLOS Prediction Model
This study found a highly accurate PLOS prediction score. A cut-off score of > 2 was associated with a high probability of mortality (> 84%). The score had excellent discrimination performance and calibration. Intraoperative vasopressor/inotropic usage, low GCS, respiratory failure, kidney failure, and intraoperative PRC transfusion were identified as predictive mortality factors in ICU. The use of vasopressor/inotropic consistenly had high score in predicting both mortality and PLOS in our population.
Several studies found various predictors of ICU PLOS including age, comorbidity, prolonged mechanical ventilation, sepsis and laboratory finding. Renal failure, the use of vasopressor/inotropic, and mechanical ventilator are the most predictor found in previous studies.22–25
APACHE IV seems to have less accuracy to predict PLOS in ICU despite the good discrimination and calibration in predicting mortality in our population. The AUC for predicting PLOS is 0.68 (0.62–0.74) and p value =0.01 for Hosmer-Lemeshow test for calibration, which considered poor.5
Different study design, sample size, population characteristics, operational definitions, inclusion and exclusion criteria and different methods of modelling may affect the results. Some studies used different definitions for prolonged ICU length of stay varied, for instance 14 days in Tobi and Amadasun, 30 days in Cevic et al. For the current study, we defined ICU prolonged length of stay as >7 ICU days, similar used in a study by Bohmer et al.25 Despite different characteristic of the population, methods, and operational definition of PLOS we found common variables predicting PLOS in ICU.
5. CONCLUSION
Perioperative vasoactive/inotropic agents support, neurological disorder, respiratory failure, kidney failure, and perioperative blood transfusion were predictors of ICU mortality. Meanwhile, medical cases, GCS <8, vasoactive/inotropic support, sepsis, respiratory failure, kidney failure, and transfusion were predictors of ICU PLOS. Both ICU mortality and PLOS score had excellent discrimination performance.
6. Data availability
Numerical data generated in the course of this study is available with the correspondence author.
7. Acknowledgements
A part of this study utilized data from the theses of Rizky Ahmad Fauzy and Firman Ardiansyah.
8. Conflicts of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
9. Authors’ contribution
All authors contributed in the preparation of the study protocol, conduct of the study, literature search and manuscript preparation.
10. REFERENCES
- Rao MH, Marella P, Kath B. Assessment of Severity and Outcome of Critical Illness. Indian J Anaesth. 2022;52(Suppl (5)):652–62.
- Zimmerman JE, Kramer AA, McNair DS, Malila FM. Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today's critically ill patients. Crit Care Med. 2006;34(5):1297-310. [PubMed] DOI: 1097/01.CCM.0000215112.84523.F0
- Vasilevskis EE, Kuzniewicz MW, Cason BA, Lane RK, Dean ML, Clay T, et al. Mortality Probability Model III and Simplified Acute Physiology Score II: assessing their value in predicting length of stay and comparison to APACHE IV. Chest. 2009 Jul;136(1):89–101. [PubMed] DOI: 1378/chest.08-2591
- Lee H, Shon YJ, Kim H, Paik H, Park HP. Validation of the APACHE IV model and its comparison with the APACHE II, SAPS 3, and Korean SAPS 3 models for the prediction of hospital mortality in a Korean surgical intensive care unit. Korean J Anesthesiol. 2014 Aug;67(2):115–22. [PubMed] DOI: 4097/kjae.2014.67.2.115
- Widyastuti Y, Zaki WA, Widodo U, Jufan AY, Pratomo BY. Predictive accuracy of the APACHE IV scores on mortality and prolonged stay in the intensive care unit of Dr Sardjito Hospital. Med J Malaysia. 2022 Jul;77(Suppl 1):53–8. [PubMed]
- Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15(4):361–87. [PubMed] DOI: 1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4
- Sullivan LM, Massaro JM, D’Agostino RB. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat Med. 2004;23(10):1631–60. [PubMed] DOI: 1002/sim.1742
- Nielsen AB, Thorsen-Meyer HC, Belling K, Nielsen AP, Thomas CE, Chmura PJ, et al. Survival prediction in intensive-care units based on aggregation of long-term disease history and acute physiology: a retrospective study of the Danish National Patient Registry and electronic patient records. Lancet Digit Health. 2019 Jun;1(2):e78–89. [PubMed] DOI: 1016/S2589-7500(19)30024-X
- Sviri S, Hashoul J, Stav I, van Heerden PV. Does high-dose vasopressor therapy in medical intensive care patients indicate what we already suspect? J Crit Care. 2014;29(1):157–60. [PubMed] DOI: 1016/j.jcrc.2013.09.004
- Auchet T, Regnier MA, Girerd N, Levy B. Outcome of patients with septic shock and high-dose vasopressor therapy. Ann Intensive Care. 2017 Apr 20;7:43. [PubMed] DOI: 1186/s13613-017-0261-x
- Marshall JC, Cook DJ, Christou NV, Bernard GR, Sprung CL, Sibbald WJ. Multiple organ dysfunction score: a reliable descriptor of a complex clinical outcome. Crit Care Med. 1995 Oct;23(10):1638–52. [PubMed] DOI: 1097/00003246-199510000-00007
- Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707–10. [PubMed] DOI: 1007/BF01709751
- Le Gall JR. A New Simplified Acute Physiology Score (SAPS II) Based on a European/North American Multicenter Study. JAMA. 1993;270(24):2957. [PubMed] DOI: 1001/jama.270.24.2957
- Xing XZ, Wang HJ, Huang CL, Yang QH, Qu SN, Zhang H, et al. Prognosis of patients with shock receiving vasopressors. World J Emerg Med. 2013;4(1):59–62. [PubMed] DOI: 5847/wjem.j.issn.1920-8642.2013.01.011
- Overgaard CB, Dzavík V. Inotropes and vasopressors: review of physiology and clinical use in cardiovascular disease. Circulation. 2008;118(10):1047–56. [PubMed] DOI: 1161/CIRCULATIONAHA.107.728840
- Bellomo R, Giantomasso DD. Noradrenaline and the kidney: friends or foes? Crit Care. 2001;5(6):294–8. [PubMed] DOI: 1186/cc1052
- Bello AK, Alrukhaimi M, Ashuntantang GE, Basnet S, Rotter RC, Douthat WG, et al. Complications of chronic kidney disease: current state, knowledge gaps, and strategy for action. Kidney Int Suppl. 2017;7(2):122–9. [PubMed] DOI: 1016/j.kisu.2017.07.007
- Thomas R, Kanso A, Sedor JR. Chronic kidney disease and its complications. Prim Care. 2008 Jun;35(2):329–44, vii. [PubMed] DOI: 1016/j.pop.2008.01.008
- Nik A, Sheikh Andalibi MS, Ehsaei MR, Zarifian A, Ghayoor Karimiani E, Bahadoorkhan G. The Efficacy of Glasgow Coma Scale (GCS) Score and Acute Physiology and Chronic Health Evaluation (APACHE) II for predicting hospital mortality of ICU patients with acute traumatic brain injury. Bull Emerg Trauma. 2018;6(2):141–5. [PubMed] DOI: 29252/beat-060208
- Hébert PC, Wells G, Tweeddale M, Martin C, Marshall J, Pham B, et al. Does transfusion practice affect mortality in critically ill patients? Transfusion Requirements in Critical Care (TRICC) Investigators and the Canadian Critical Care Trials Group. Am J Respir Crit Care Med. 1997 May;155(5):1618–23. [PubMed] DOI: 1164/ajrccm.155.5.9154866
- Napolitano LM, Corwin HL. Efficacy of red blood cell transfusion in the critically ill. Crit Care Clin. 2004;20(2):255–68. [PubMed] DOI: 1016/j.ccc.2003.12.002
- Peres IT, Hamacher S, Oliveira FLC, Thomé AMT, Bozza FA. What factors predict length of stay in the intensive care unit? Systematic review and meta-analysis. J Crit Care. 2020;60:183–94. [PubMed] DOI: 1016/j.jcrc.2020.08.003
- Çevik B, Geyik FD. Prolonged stay in intensive care unit: retrospective analysis of predisposing factors and outcome. Turk J Intensive Care. 2019 Jun 1;17(2):96–101. DOI: 4274/tybd.galenos.2018.58561
- Toptas M, Sengul Samanci N, Akkoc İ, Yucetas E, Cebeci E, Sen O, et al. Factors Affecting the Length of Stay in the Intensive Care Unit: Our Clinical Experience. BioMed Res Int. 2018;2018:9438046. [PubMed] DOI: 1155/2018/9438046
- Böhmer AB, Just KS, Lefering R, Paffrath T, Bouillon B, Joppich R, et al. Factors influencing lengths of stay in the intensive care unit for surviving trauma patients: a retrospective analysis of 30,157 cases. Crit Care. 2014;18(4):R143. [PubMed] DOI: 10.1186/cc13976